
November 6, 2017
Salk researchers discover that cutting off energy production in regulatory T cells impairs their function
LA JOLLA—Regulatory T cells (Tregs) are the traffic cops of the immune system. They instruct other types of immune cells on when to stop and when to go. Learning how to direct the activity of Tregs has important implications for improving cancer immunotherapy as well as developing better treatments for autoimmune diseases such as rheumatoid arthritis and type 1 diabetes.

Click here for a high-resolution image
Credit: Salk Institute
Now, a research team from the Salk Institute has uncovered for the first time a protein that controls both the survival and function of Tregs. The discovery, published in the Proceedings of the National Academy of Sciences the week of November 6, 2017, suggests ways to influence the function of Tregs and ultimately to make immune-related therapies more effective.
“Tregs are at the crossroads of inflammation,” says senior author Ronald Evans, Howard Hughes Medical Institute investigator and holder of Salk’s March of Dimes Chair in Molecular and Developmental Biology. “If you have a lot of Tregs in the environment, they weaken your immune response. If you have too few you go down the road of chronic inflammation.”
“Right now there are no good targets for controlling Tregs,” says Nanhai He, a research associate in Evans’ laboratory and the study’s first author. “This finding is very new and very important, because it shows us the role of cellular metabolism in how these immune cells function.”
The protein the team studied is called Lkb1 (for liver kinase B1). Kinases are enzymes that catalyze reactions inside cells. Lkb1 was previously known to play a role in cell metabolism, but until this study, investigators didn’t know that it controls the functions in the immune response of Tregs.
“When we talk about metabolism, most people think about factors like what we eat and how much we exercise,” says Annette Atkins, a staff researcher in Evans’ lab. “But in this case, we’re looking at the metabolism of individual cells. By compromising the ability of these cells to make energy, we see very profound autoimmune disorders.”
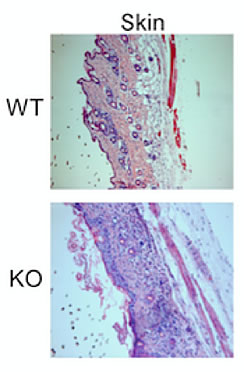
In the current study, the team used mouse models that had the Lkb1 gene knocked out in their regulatory T cells. The mice showed many symptoms of autoimmune disease and died within a few weeks of birth. Further examination revealed that the normal metabolic machinery in the Tregs was disrupted. The cells had defective mitochondria—cellular power stations—and depleted levels of ATP, which is their most important fuel source.
“Through these observations, we determined that the Lkb1 pathway is responsible for supplying Tregs with energy,” says Ye Zheng, an associate professor in Salk’s Nomis Foundation Laboratories for Immunobiology and Microbial Pathogenesis. “Without it, Tregs don’t have enough fuel to function.”
Credit: Salk Institute
“It turns out that Tregs require a lot of energy to do their job, which is essentially to prevent other kinds of T cells from attacking the body,” adds Michael Downes, a Salk senior scientist. “This is something that wasn’t previously recognized, and it’s an important discovery.”
The investigators say the findings have implications for both cancer immunotherapy and therapy for autoimmune diseases.
In cancer, Tregs are recruited by tumors and prevent other types of T cells, including cytotoxic T cells (also called CD8 cells) from attacking and destroying cancer cells. “To boost cancer immunotherapy, we’d like to find ways to block the Lkb1 pathway,” Zheng explains. “The outcome of this inhibition would be an increased immune response from other types of T cells, which would help them to destroy tumors.”
On the other hand, boosting the ability of Tregs to suppress other types of immune cells could prevent autoimmunity, by preventing these cells from attacking organs and other tissues. Boosting the Treg population also has potential to avert immune rejection after an organ transplant.
The investigators say that although Lkb1 itself is difficult to target, they have already identified molecules downstream in the signaling pathway that could be altered with drugs. “These drugs could either inhibit or enhance the pathway, depending on what we want them to do,” He explains. Further research from the team will focus on the development of such drugs.
Other researchers involved in the study were Weiwei Fan, Brian Henriquez and Ruth Yu of Salk; and Christopher Liddle of the University of Sydney.
This work was funded by the National Institutes of Health, the National Institute of Environmental Health Sciences, the Leona M. and Harry B. Helmsley Charitable Trust, the Foundation Leducq and Ipsen/Biomeasure.
JOURNAL
Proceedings of the National Academy of Sciences
AUTHORS
Nanhai He, Weiwei Fan, Brian Henriquez, Ruth T. Yu, Annette R. Atkins, Christopher Liddle, Ye Zheng, Michael Downes, and Ronald M. Evans
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.