American Cancer Society Professor
Molecular and Cell Biology Laboratory
Renato Dulbecco Chair


Cells are like creatures of habit—they follow the same cellular cycle over and over, coordinating the timing of gene and protein activation with growth and division. If this cycle is broken, things start to fall apart: Cells begin copying the wrong genes, turning on proteins at the wrong times or dividing too quickly or too slowly. All of these disruptions can lead to cancer. Understanding how a healthy cell controls its growth cycle can help researchers get a better grasp on what goes wrong in tumor cells when their growth spirals out of control—and how to fix it. But it’s hard to pinpoint which individual genes and proteins are most important.
Tony Hunter made the seminal discovery, more than four decades ago, that the addition and subtraction of phosphate molecules to proteins on tyrosine, one of the 20 amino acids, allows cells to control when key proteins are on standby and when they are active. He went on to show that, in cancers, growth was switched to an always-on mode by the malfunctions of these phosphates. Since then, his lab has led the field in understanding how chemical additions to proteins control the cell cycle and growth. Hunter uses cutting-edge molecular, genetic and cell biology techniques to probe how these programs interact with each other, what effect they have on cells and how cancers disrupt them to encourage uninhibited growth.
Already, cancer drugs—such as the leukemia therapy Gleevec™—have been designed based on Hunter’s discoveries. Gleevec turns off an enzyme that normally adds phosphates to tyrosines in proteins, thus preventing cancers from growing. As Hunter continues to discover other ways in which cells use chemical additions to proteins to control their growth, he aims to find potential therapeutic targets for cancers.
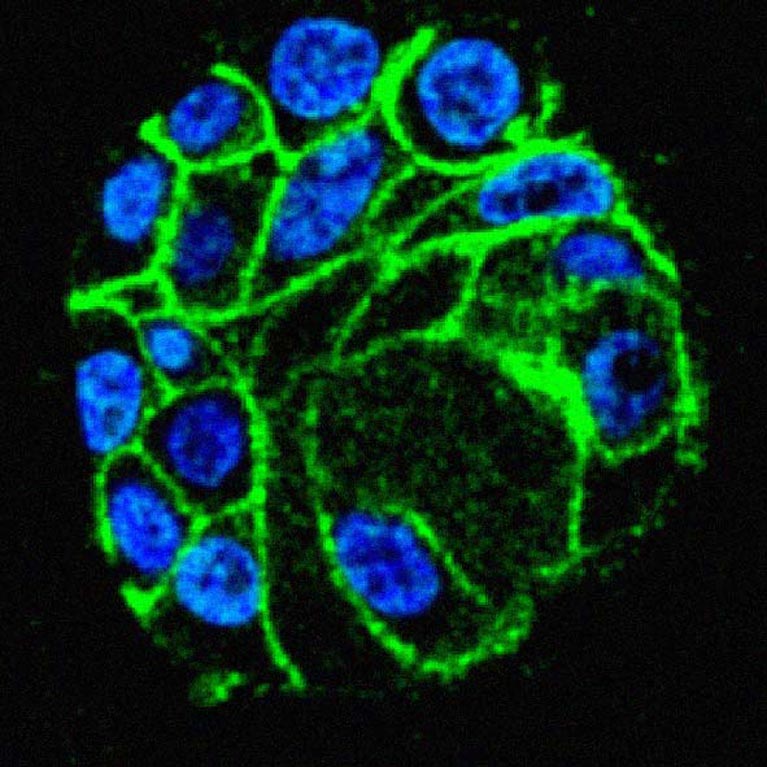
Hunter demonstrated that a mechanism called tyrosine phosphorylation (the addition of phosphate molecules to an amino acid in proteins) acts as a master on/off switch for a number of key proteins. This discovery has led to new, successful cancer therapies.
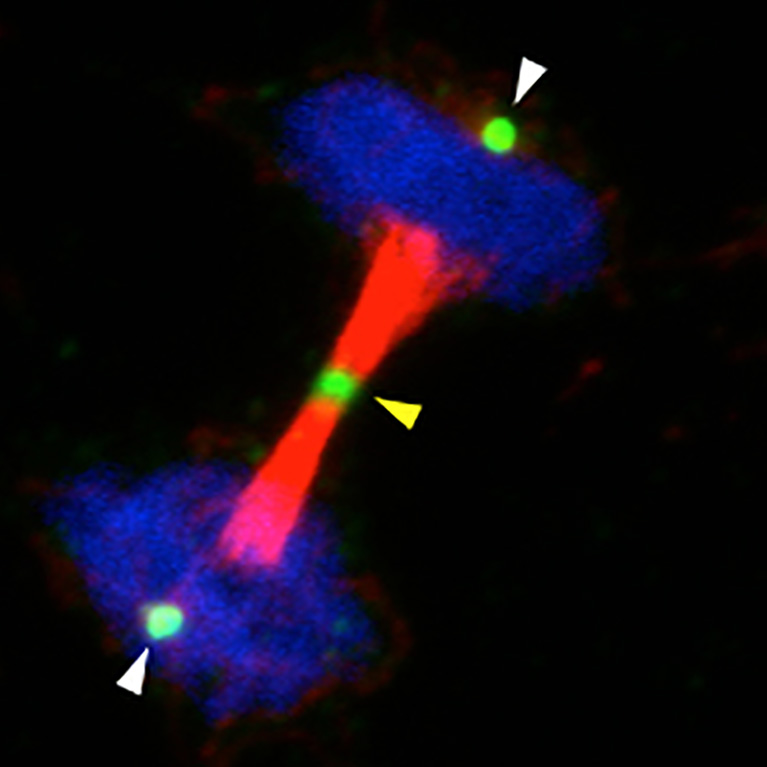
Hunter helped to explain precisely how cells mobilize their repair crews to fix damaged DNA, an important mechanism for preventing cells from turning cancerous.
Hunter showed how some cancers find a loophole in the cellular security system that should destroy them, which helps them to recover and resume dividing after treatment with DNA- damaging cancer drugs. In pancreatic cancer, drugs cannot reach the tumor due to an inflammatory barrier created by crosstalk between the tumor and pancreatic cells, but Hunter has found a way disrupt this communication via a signaling molecule called LIF. LIF could be a useful biomarker or target for the treatment of pancreatic cancer.
BA, First Class Honors, University of Cambridge, England
PhD, University of Cambridge, England
Postdoctoral Fellow, The Salk Institute and University of Cambridge