Professor
Clayton Foundation Laboratories for Peptide Biology
Helen McLoraine Chair in Molecular Neurobiology

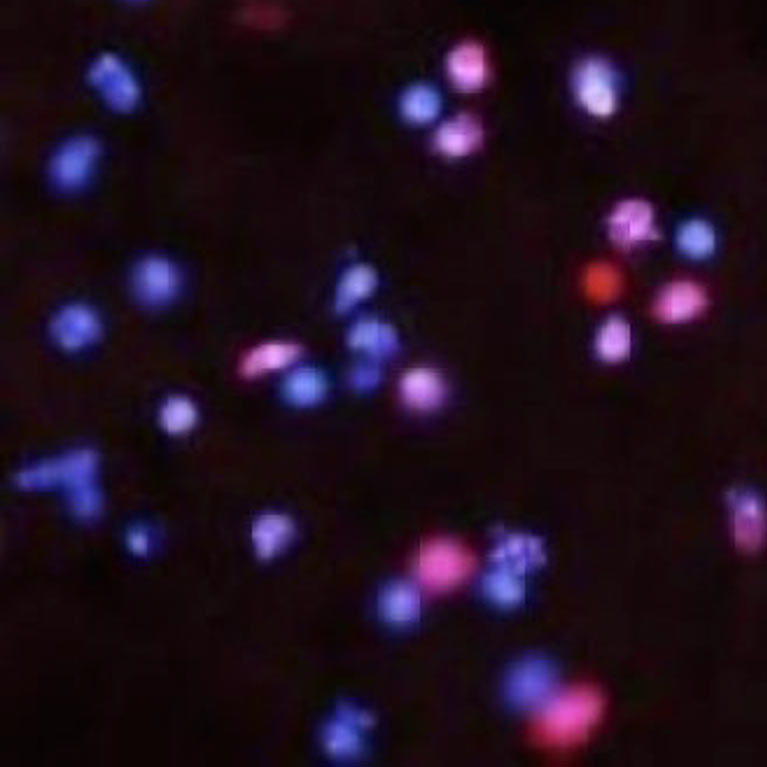
How can we extend lifespan while preventing age-related diseases like Alzheimer’s and Parkinson’s? These neurodegenerative disorders are the result of many years of cumulative, complex events in the brain. The pre-dementia phase of Alzheimer’s can sometimes last a full 20 years before dementia is clinically diagnosed. The perplexing observation that shapes Lee’s research is that some neurons and non-neuronal cells seem more vulnerable to degeneration while others stand strong, a phenomenon neuroscientists call “selective vulnerability and resilience.” Unlike current approaches that focus on managing symptoms after disease onset, Lee is working to understand how brain cells become vulnerable to the neurodegenerative events that trigger the disease to begin with. Lee’s research paves the way for future therapeutics that can detect and prevent age-related diseases and promote longer, healthier lives.
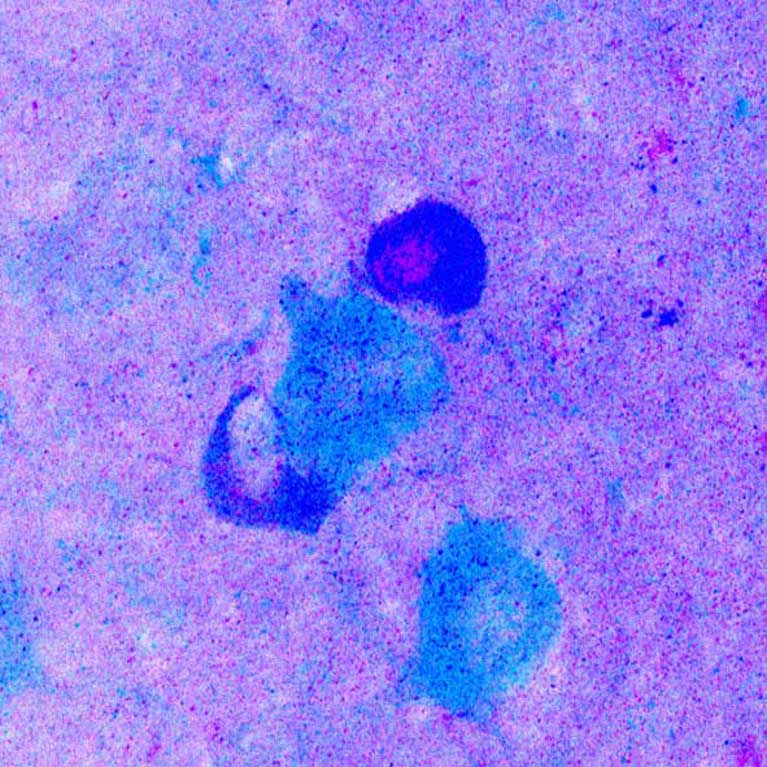
Lee looks at how genetic, environmental, and lifestyle factors intersect to influence healthy aging and disease progression. When these factors converge in the brain, they create a complex, interconnected web of impacted cells and processes to untangle.
The brain contains both neurons and non-neuronal support cells, both of which are indispensable for healthy function. Between each neuron are connective junctions called synapses, where information is passed from one cell to the next. These synaptic connections are the basis for brain circuitry. For Lee, untangling these cells and circuits happens through an artificial intelligence (AI)-enabled multi-scale and multi-modal approach that integrates comparative and functional genomics across multiple species and models. Once untangled, the mechanisms of selective vulnerability and resilience in aging and Alzheimer’s will reveal themselves and enable new forms of mechanisms-based precision medicine.
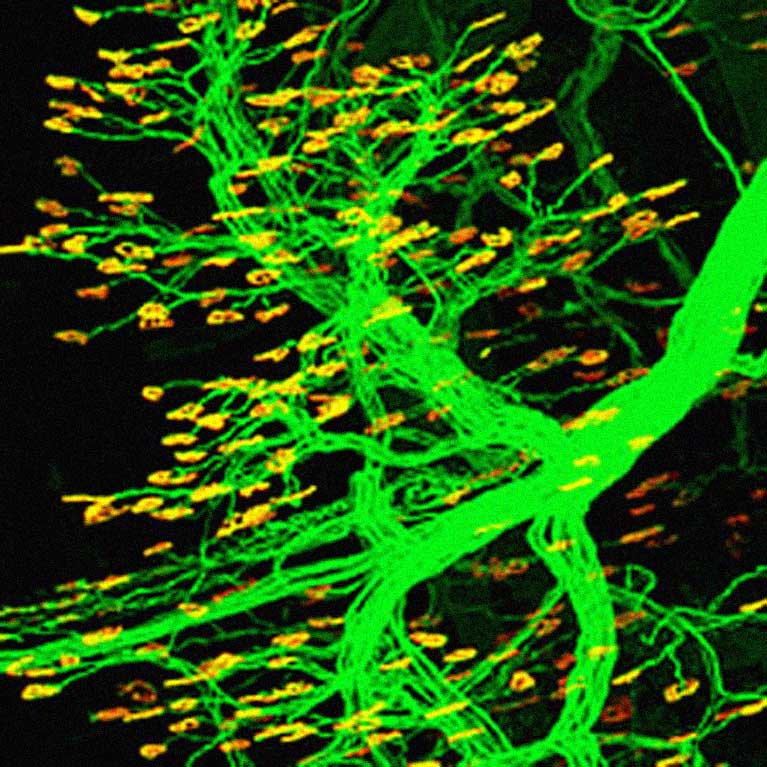
Lee discovered that physical stress and anxiety-inducing hormones contribute to tau protein modifications that can lead to tau tangles in Alzheimer’s, and that trophic factors can relieve neuropathology and cognitive impairment in Alzheimer’s disease models.
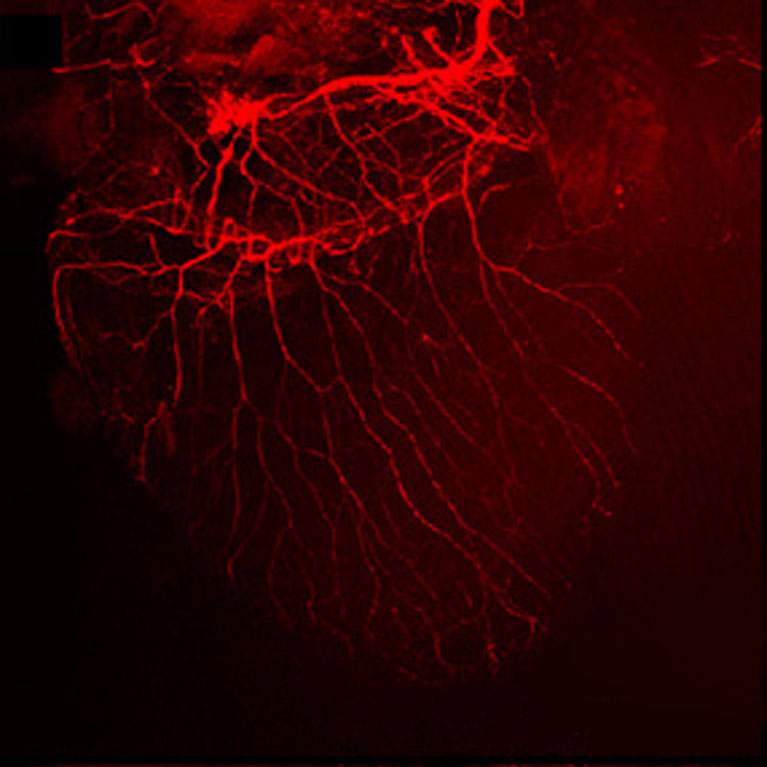
Lee used AI-detected behavioral deficits to identify several selectively vulnerable cell types and metabolic pathways in the predementia stage of Alzheimer’s disease.
Lee has identified new and novel brain circuits and molecular targets underlying Parkinsonian motor and neuropsychiatric symptoms.
Plant Pathology, National Taiwan University
MS, Cancer Enzymology and Cell Differentiation, National Yang-Ming Medical College, Taiwan
PhD, Endocrinology, Baylor College of Medicine, Houston
Postdoctoral Fellow, Whitehead Institute for Biomedical Research