
February 9, 2009
"Everybody in the world is my friend"
"Everybody in the world is my friend"
La Jolla, CA–Unraveling the genetics of social behavior and cognitive abilities, researchers at the University of Utah and the Salk Institute for Biological Studies have traced the role of two genes,GTF2I and GTF2IRD, in a rare genetic disorder known as Williams Syndrome.
Their results, published in the Feb. 9, 2009, online edition of the American Journal of Medical Genetics, suggest that GTF2IRD1 contributes to visual-spatial performance while GTF2I plays a role in social behavior. These findings illuminate the most complex aspects of being human.
“Identifying these two genes is the pinnacle of many years’ work examining hundreds of cases with Williams syndrome and developing techniques and analyses to find individual genes associated with behavior,” says team leader Julie R. Korenberg, M.D., Ph.D., professor and director of the Center for Integrated Neurosciences and Human Behavior at the Brain Institute at the University of Utah and Salk Institute adjunct professor. Dr. Korenberg has studied Williams syndrome for more than 15 years through a Program Project from NICHD called “Williams Syndrome: Linking Cognition, Brain and Gene.” Collaborating on the behavioral aspects of this disorder has been Ursula Bellugi, professor and director of the Laboratory of Cognitive Neuroscience at the Salk Institute.
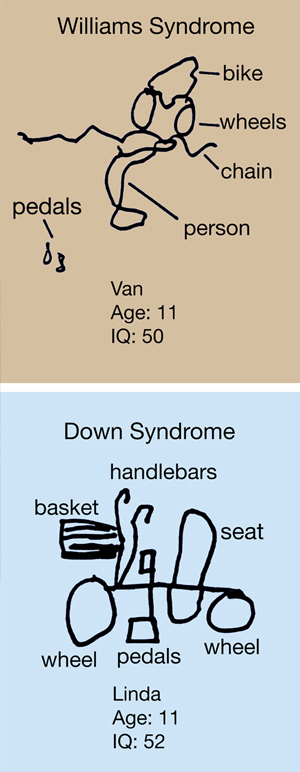
TOP: Asked to draw a bicycle, children with Williams syndrome will show all the parts, but strew them randomly across the page. BOTTOM: A child with Down syndrome will come up with something simple but recognizable.
Image: Courtesy of Dr. Ursula Bellugi, Salk Institute for Biological Studies.
To children with Williams Syndrome, people are much more comprehensible than inanimate objects. Despite myriad health problems and generally low IQs, these patients are extremely gregarious, irresistibly drawn to strangers, and insist on making eye contact. The children are confounded by the visual world around them, however: Asked to draw a bicycle, they will show all the parts, but strew them randomly across the page (see figure). This strange mix of mental peaks and valleys drew Korenberg and Bellugi to the disorder.
“Williams syndrome presents a unique lens for discovering the how genes form the brain circuitry responsible for human behavior,” explains Korenberg. This prompted Korenberg’s collaboration with Bellugi, who is interested in the cognitive aspects of the disorder, “Genetic contributions to human cognition and behavior are clear but difficult to define,” says Bellugi.
Virtually everyone with Williams Syndrome has exactly the same set of genes missing (25 to 28 genes are missing from one of two copies of chromosome 7). There also are rare cases of individuals who retain one or more genes that most people with the disorder have lost.
“I wanted to focus on something that could get me down to just a few genes related to behavior,” says senior author Korenberg, also a USTAR professor of pediatric genetics at the University of Utah School of Medicine. “When it became clear that Williams Syndrome is caused by a microdeletion of genes, we could start looking at smaller and smaller gene deletions to study their effect on social behavior.”
Two genes in particular, GTF2IRD1 and GTF2I, caught Korenberg’s eye. Both encode transcription factors that help regulate the activity of other genes. Although their exact function is unknown, the genes are active in many body tissues and appear to be particularly important in regulating brain and skeletal muscle genes.
In earlier studies, Korenberg and her collaborators linked both GTF2I and GTF2IRD1 to deficits in visual-spatial processing, a hallmark of Williams Syndrome. The researchers are now dissecting the genes’ roles even more. “Further parsing the effects of GTF2IRD1 versus GTF2I on spatial construction and social behavior was previously hampered by the small number of cases with fewer than the usual gene deletions and limited cognitive data,” explains Korenberg.
To distinguish the roles of the two genes, postdoctoral researcher and study first author Li Dai, Ph.D., combed the genomes of 17 Williams Syndrome patients to identify those who had lost only one GTF2I gene. This allowed identification of a girl who had retained GTF2I but didn’t fit the classical description of the disorder. “Finding this girl was very exciting,” Korenberg said. “Her case had so much power to explain the role of these genes.”
When the Salk researchers tested the girl to measure her IQ and social behavior, they found her scores in vocabulary, information processing, comprehension, arithmetic, and the ability to finish partially completed drawings to be substantially closer to normal than most patients with Williams syndrome.
Her full-spectrum IQ, a measure of both functional and performance intelligence, was 78, a full 18 points higher than average for someone with the disorder. Yet in two areas, the ability to assemble objects or work through a maze, the girl scored lower than average for people with the syndrome and substantially lower than normal.
These tests also confirmed that her social behavior is different than expected. While she is charming and engaging, she does not run up to people and does not maintain as much eye contact or physical proximity to others when conversing.
“Because she has the typical facial features and severe deficits in visual spatial skills, but lacks the overly social behavior, it suggested to us that GTF2IRD1 contributes to visual-spatial performance while GTF2I plays a role in social behavior,” says Korenberg.
Although this work presents a major step forward in linking GTF2I to social behavior, it does not mean they are the only genes involved, Korenberg notes. Endowed with the power to control the activity of other genes, GTF2I might regulate signal pathways determining the structure and function of the brain or the production of neurohormones such as vasopressin and oxytoxin. Oxytoxin plays a key role in the desire to seek social interactions and trusting others, which might explain why for children with Williams syndrome, the world has no strangers, only friends.
About the Brain Institute at the University of Utah:
One in three people will suffer from a brain disorder during their lifetime. The other two thirds will suffer right along with them. The Brain Institute at the University of Utah, is doing something about that. Born in 2005 with the vision and support of the University of Utah leadership, more than 100 scientists, researchers, practitioners, and administrators are dedicated to advancing the mission of The Brain Institute.
In a short period of time, many nationally and internationally recognized experts have embraced the goals of The Brain Institute, furthering their research in Alzheimer’s Disease, Autism, Depression, Multiple Sclerosis, Parkinson’s, Spinal Cord Injuries, Substance Abuse, and Stroke.
As a hub of U.S. genetic research, Salt Lake City and the intermountain area provide a unique benefit to The Brain Institute: a receptive environment and many established centers that provides the perfect home and resources for leadership in the research and understanding of every type of devastating brain disorder.
About the Salk Institute:
The Salk Institute for Biological Studies in La Jolla, California, is an independent nonprofit organization dedicated to fundamental discoveries in the life sciences, the improvement of human health, and the training of future generations of researchers. Jonas Salk, M.D., whose polio vaccine all but eradicated the crippling disease poliomyelitis in 1955, opened the Institute in 1965 with a gift of land from the City of San Diego and the financial support of the March of Dimes.
Office of Communications
Tel: (858) 453-4100
press@salk.edu