
April 15, 2021
The findings on cellular communication hold promise for early human development, disease progression and aging, as well as organ transplantation and for testing therapeutics
The findings on cellular communication hold promise for early human development, disease progression and aging, as well as organ transplantation and for testing therapeutics
LA JOLLA—The ability to grow the cells of one species within an organism of a different species offers scientists a powerful tool for research and medicine. It’s an approach that could advance our understanding of early human development, disease onset and progression and aging; provide innovative platforms for drug evaluation; and address the critical need for transplantable organs. Yet developing such capabilities has been a formidable challenge.
Researchers led by Salk Professor Juan Carlos Izpisua Belmonte have now come one step closer toward this goal by demonstrating a new integration of human cells into animal tissue. Published in the journal Cell on April 15, 2021, the new study builds upon previous work by the Izpisua Belmonte lab to take the next step in chimeric organisms—organisms that contain cells from two or more species—to understand a host of diseases and address the severe shortage in donor organs.
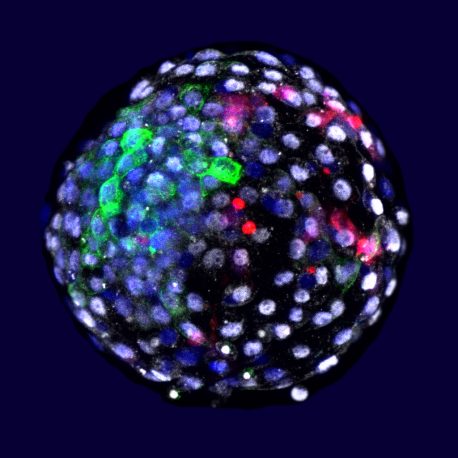
“These chimeric approaches could be really very useful for advancing biomedical research not just at the very earliest stage of life, but also the latest stage of life,” says Izpisua Belmonte.
The World Health Organization estimates that the 130,000 organ transplants performed every year represent only 10 percent of the need, which is exacerbated by a shortage of available organs. Researchers hoped that growing human cells in pig tissue—whose organ size, physiology and anatomy are similar to humans’—could alleviate this problem. Previously, in a 2017 Cell study, Izpisua Belmonte’s group reported groundbreaking work in which they incorporated human cells into early-stage pig tissue, marking the first step toward producing transplantable human organs using large animals. But the contribution of human cells was fairly low, which could be due to the large evolutionary distance (90 million years) between the two species. So Izpisua Belmonte set out to investigate chimera formation in a more closely related species, macaques.
While these types of chimeras with macaques wouldn’t be used for human organ transplants, they nevertheless reveal invaluable information about how human cells develop and integrate, and how cells of different species communicate with one another. Izpisua Belmonte likens the process of two types of cells integrating to communicating with different languages: human cells in pig tissue was akin to the cells trying to find common ground between Chinese and French for example, whereas human cells in macaques operated more like two closely related languages, like Spanish and French. By better understanding molecular pathways involved in this interspecies communication, researchers could ultimately improve the integration of human cells into more suitable hosts, such as pigs, that could be used in regenerative medicine, as well as better understanding the aging process.
In the present study, the team tagged human pluripotent stem cells (cells that are capable of developing into all the cell types in the body) with a fluorescent protein and inserted these labeled stem cells into macaque embryos in petri dishes. The current study was made possible by technology published last year by the collaborating team led by Prof. Weizhi Ji of Kunming University of Science and Technology in Yunnan, China, that allowed monkey embryos to stay alive and grow outside the body for an extended period of time. In the case of this work, all experiments were terminated 19 days after stem cell injection. Through immunofluorescent studies, in which antibodies bind to the fluorescently tagged stem cells, the scientists observed that human stem cells survived and integrated with better relative efficiency than in the previous experiments in pig tissue.
To identify the molecular communication pathways between the two species’ cells in the current study, Izpisua Belmonte’s lab analyzed the chimeric transcriptome, a readout of which genes and molecules are active. They observed that cells from chimeric tissue had distinct transcriptomic profiles from controls and detected several communications pathways that were strengthened or novel in the chimeric cells.
Once this molecular communication is further understood, chimeric organisms could allow researchers an unprecedented glimpse into the earliest stages of human development. Chimeric organisms containing human cells could be used to generate cells and organs for transplantations in host species more evolutionarily distant to humans, like pigs, that might be more appropriate for various reasons (social, economic and ethical, among others). Additionally, these studies constitute a new platform to study how specific diseases arise. For instance, a particular gene that may be associated with a certain cancer could be engineered in a human cell. Then, observing the course of disease progression using those engineered cells in a chimeric model might reveal more applicable results than a typical animal model in which the disease might take a different course. Chimeric models of disease could also be used to test the efficacy of drug compounds and obtain results that could similarly better reflect the response in humans.
Another research avenue where chimerism could offer unique insights is aging. Izpisua Belmonte says researchers don’t know if organs age at the same rate or if perhaps one organ drives the aging of all the other organs and acts as a master switch for the aging process. Using chimerism to grow, for example, the organ of a common rat in a much longer-lived species like the naked mole rat, scientists could begin to probe which organs may be key to aging and what signals are involved in their survival.
As a leading expert in the field of chimera research, Izpisua Belmonte consulted all the appropriate regulatory bodies as well as independent bioethicists to ensure their work met current ethical and legal guidelines.
Next, Izpisua Belmonte plans to take a closer look at the molecular pathways the team identified as involved in interspecies communication and determine which ones are critical to the success of this process.
Other authors included: Reyna Hernandez-Benitez, W. Travis Berggren, May Schwarz and Concepcion Rodriguez Esteban of the Salk Institute; Llanos Martinez Martinez and Estrella Nuñez Delicado of the Universidad Católica San Antonio de Murcia; Jun Wu now at the University of Texas Southwestern Medical Center; Tao Tan, Chenyang Si, Shaoxing Dai, Youyue Zhang, Nianqin Sun, E. Zhang, Honglian Shao, Wei Si, Pengpeng Yang, Hong Wang, Zhenzhen Chen, Ran Zhu, Yu Kang, Zongyong Ai, Tianqing Li, Weizhi Ji and Yuyu Niu of the Kunming University of Science and Technology.
DOI: 10.1016/j.cell.2021.03.020
JOURNAL
Cell
AUTHORS
Tao Tan, Jun Wu, Chenyang Si, Shaoxing Dai, Youyue Zhang, Nianqin Sun, E. Zhang, Honglian Shao, Wei Si, Pengpeng Yang, Hong Wang, Zhenzhen Chen, Ran Zhu, Yu Kang, Reyna Hernandez-Benitez, Llanos Martinez Martinez, Estrella Nuñez Delicado, W. Travis Berggren, May Schwarz, Zongyong Ai, Tianqing Li, Concepcion Rodriguez Esteban, Weizhi Ji, Yuyu Niu, Juan Carlos Izpisua Belmonte
Office of Communications
Tel: (858) 453-4100
press@salk.edu
The Salk Institute is an independent, nonprofit research institute founded in 1960 by Jonas Salk, developer of the first safe and effective polio vaccine. The Institute’s mission is to drive foundational, collaborative, risk-taking research that addresses society’s most pressing challenges, including cancer, Alzheimer’s, and agricultural resilience. This foundational science underpins all translational efforts, generating insights that enable new medicines and innovations worldwide.