2025: The Salk Institute’s Year of Alzheimer’s Disease Research
Salk scientists have long been investigating the many molecular hallmarks of aging, with support from the American Heart Association and Allen Frontiers Group Initiative. From this experience, the team has homed in on chronic inflammation as a critical driver of Alzheimer’s disease.
The challenge
Alzheimer’s disease is one of the most significant public health crises of our time. Despite more than $30 billion in research funding since 1984, no cure, prevention, or effective long-term treatment exists.
It’s no secret that Alzheimer’s research hasn’t progressed as quickly as we—and the many families affected—would like. That’s in part due to tunnel vision.
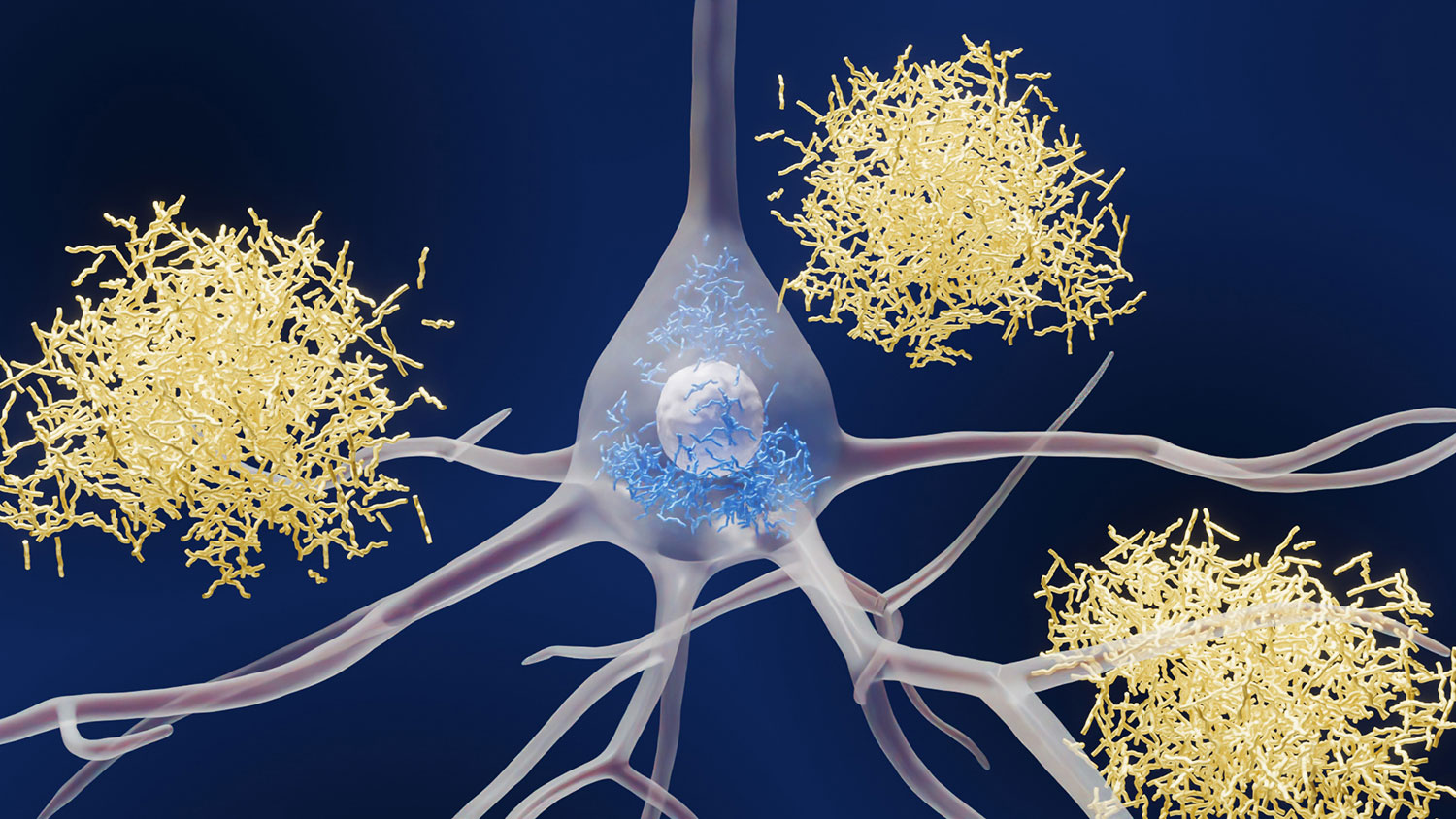
Most Alzheimer’s research has focused on the plaques (white) and tangles (dark blue) that can form in and around neurons (light blue) at later stages of the disease.
Focus almost exclusively on neurons. Most research on brain function and cognitive decline is focused exclusively on neurons. Yet, in reality, neurons make up only half of the brain’s total cell count. The other half are glial cells, which support and protect neurons and undoubtedly play an underappreciated role in maintaining brain health.
Overemphasis on amyloid plaques and tau tangles. These abnormal clumps of proteins in the brain were first observed by Alois Alzheimer himself in 1906 and thus became the defining biomarkers of the disease.
For decades, these proteins were the focus of nearly all Alzheimer’s research and more than 400 failed clinical trials.
There are two recently approved monoclonal antibody therapies that reduce amyloid plaques, but the benefit to patients is modest at best.
More attention given to rarer form of Alzheimer’s. Most Alzheimer’s research has focused on the familial type, which is a rare, early-onset form caused by inherited gene mutations. Yet more than 90% of cases are sporadic, not inherited.
The need for a transformative approach has never been more urgent.
The Salk Institute is rewriting the narrative of Alzheimer’s disease research
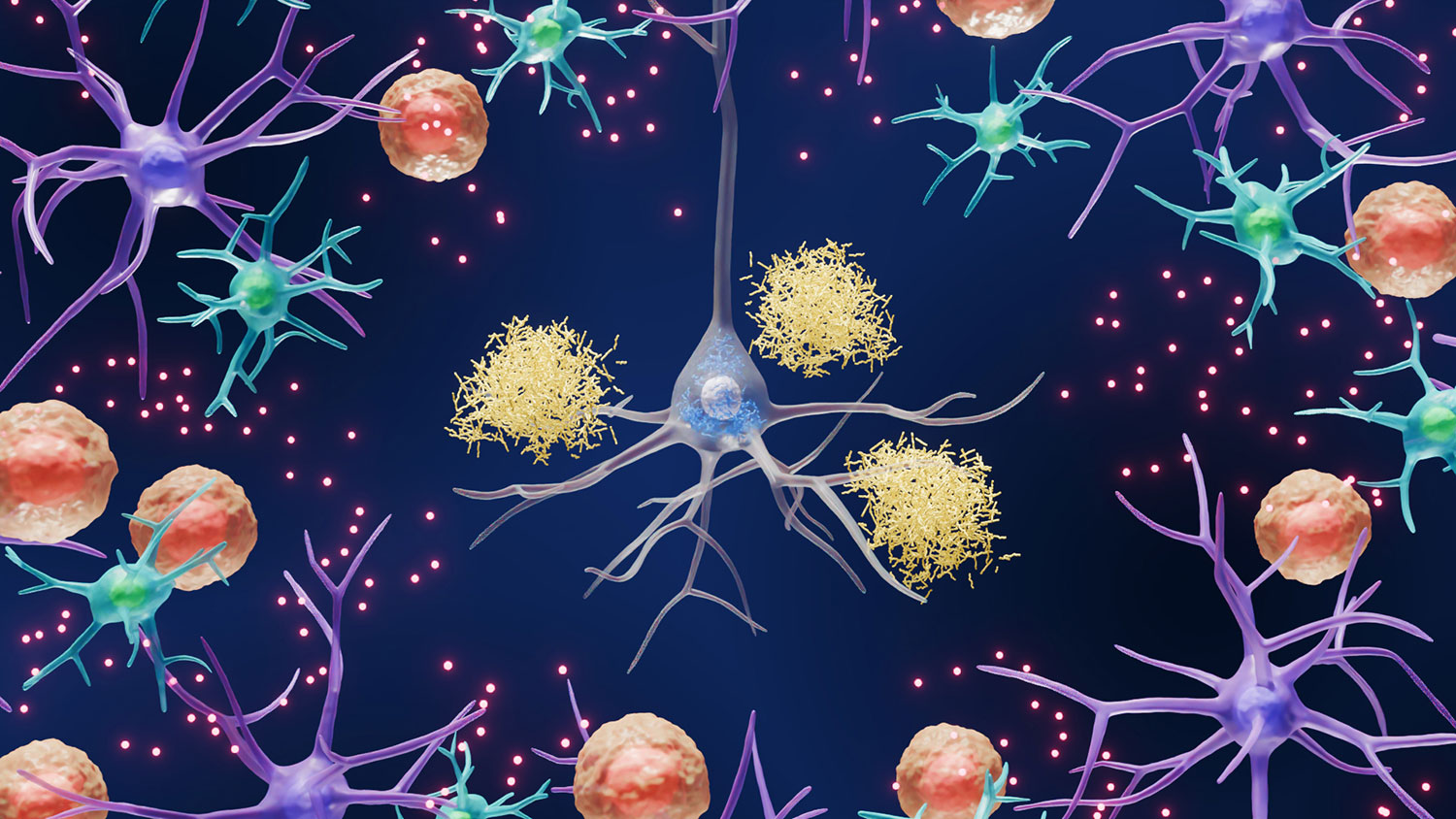
Salk’s approach pulls back the lens to consider other potential drivers of disease, including astrocytes (purple), microglia (cyan), immune cells (orange), inflammation (red), and more.
Chronic inflammation: A hidden threat in the brain. Chronic inflammation is a sustained immune response in the brain over an individual’s lifetime, and that long-term damage can ultimately manifest as Alzheimer’s disease. The Salk team also identified two key factors as key contributors to this harmful inflammation: genome instability and dysregulated energy metabolism.
Salk scientists believe that inflammation, genome stability, and energy metabolism are directly connected and influence one another.
Even at baseline, human brains require enormous amounts of energy—approximately 20% of the body’s total energy consumption, about twice that of other primates. Our brains are already running in energetic hyperdrive. So, when life throws an added stress on top of that, such as the need for extra DNA repair, or as mitochondria naturally decline with age, inflammation increases. Ultimately, this stress makes the brain more susceptible to Alzheimer’s.
Salk’s approach—widening the research lens to focus on chronic inflammation throughout the brain—will allow researchers to identify biomarkers for early signs of the disease and new opportunities for intervention with next-generation therapeutics.
What drives Alzheimer’s disease?

Chronic inflammation. The body’s natural defense response to injury or infection can cause harm if it persists as chronic inflammation or is inappropriately directed.
What contributes to harmful chronic inflammation in the brain?

Genome instability. When genomes and chromosomes are unstable, errors can occur in the way our genetic information (DNA) is copied and passed on.

Dysregulated energy metabolism. If not properly regulated, the process by which our cells use mitochondria to generate energy malfunctions, leaving cells depleted and unable to maintain healthy functions.
Cross-disciplinary collaboration drives innovation
Here are just a few examples of Salk studies that demonstrate our expertise in neuroscience, immunobiology, genomics, and metabolism, as well as our strengths in thinking creatively and collaboratively to find what few others are even looking for.
Aging biology and metabolism experts discovered that when telomeres (the end caps of chromosomes) become very short with age, they communicate with mitochondria. This communication triggers a complex set of signaling pathways and initiates an inflammatory response. The team is now determining how this crosstalk between genomes, mitochondria, and inflammation plays a role in Alzheimer’s disease.
Neuroscientists found that energy-intense neuron DNA repairs are not random but instead focus on protecting certain genetic “hot spots” that appear to play a critical role in neural identity and function. As cellular energy production declines as we age, neurons struggle to repair their genomes. Accordingly, the team is now unraveling the interplay between genomes and energy metabolism to determine its role in Alzheimer’s disease.
Salk neuroscientists have also discovered that astrocytes, the most common type of glial cell, are crucial for shaping communication across the brain. They also found that in Alzheimer’s patients, astrocytes are less able to create or strengthen synapses, ultimately disrupting brain communication. The scientists are now testing whether boosting astrocyte function in the Alzheimer’s brain can restore synaptic function and delay disease progression.
Immunologists are investigating how lifelong exposure to infections and other foreign agents influences immune cell infiltration and brain inflammation, potentially contributing to the development of Alzheimer’s disease.
Salk scientists have revealed that the body has evolved mechanisms to tolerate infections by maintaining physiological function and repairing tissue damage—both of which are necessary for survival. Beyond infections, this approach reshapes our perception of health—not merely as the absence of disease but as an active, mechanistic process of maintaining resilience and endurance. Cooperative defenses hold promise for treating non-infectious diseases such as Alzheimer’s disease and other inflammatory disorders, metabolic syndromes, and cancer, and hold secrets for regenerative medicine.
These research examples demonstrate the types of cross-disciplinary explorations that can only happen at a place like Salk.
Related News
Protecting brain cells with cannabinol Read more »
More than just neurons: A new model for studying human brain inflammation Read more »
Related Podcast Episodes
Getting to the root of Alzheimer’s Listen here »
Irene Lopéz Gutiérrez advocates for interdisciplinary Alzheimer’s research Listen here »
