
April 21, 2014
By coaxing stem cells derived from a patient's own skin cells to develop into airway tissue, scientists can study the molecular basis of asthma, emphysema and other lung diseases.
By coaxing stem cells derived from a patient's own skin cells to develop into airway tissue, scientists can study the molecular basis of asthma, emphysema and other lung diseases.
LA JOLLA—Using reprogrammed skin cells, researchers have for the first time used stem cell techniques to grow fully functional assemblies of the cells that line airways leading to the lungs. The lab-grown airway tissue can now be used to study the molecular basis for lung diseases—from rare genetic disorders to common afflictions like asthma and emphysema—and test new drugs to treat the diseases.
"This work is the beginning of an exciting era and shows progress toward one day making a three-dimensional, functional lung in the laboratory and offers opportunities for testing specific therapies," says senior study author Inder Verma, a professor in Salk's Laboratory of Genetics and holder of the Irwin and Joan Jacobs Chair in Exemplary Life Science.
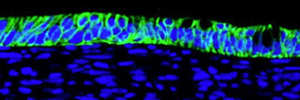
Epithelial cells (blue), which have been derived from stem cells, are arranged in a flat sheet, just as they are in the lining of the lungs. Also mimicking the way airways are arranged, the cells are contacting liquid on one side (below) and air on the other.
Image: Courtesy of the Salk Institute for Biological Studies
The results of the study were published March 21, 2014, in Proceedings of the National Academy of Sciences.
Studying diseases that affect the lungs and nearby airways has been limited by the ability to collect diseased tissue from patients and the complexity of organs. Multiple cell types make up airway tissue, so studying how a disease changes just one cell type in the lab may not be an accurate reflection of the full impact of the disease.
In the new work, the researchers developed the complex airway tissue, composed of four different cell types, by reprogramming skin cells into stem cells, then exposing the stem cells to a unique recipe of chemicals that steered them down a particular, airway-specific, developmental path. Rather than use embryonic stem cells to grow the airway tissue, they began with easily collectable skin cells from patients.
"This way, we have the ability to access patient populations with these rare genetic lung diseases," says Amy Firth, a post-doctoral researcher in Verma's lab and first author of the new paper. Since skin cells contain the same master set of genes as lung cells, any airway tissue grown from a patient's skin cells will have the disease-causing gene mutation in them.
Verma, Firth and their colleagues first used standard protocols to wipe the skin cells clean of all programming that made them skin cells, turning them into induced pluripotent stem (iPS) cells that have the potential to develop into any cell type in the body. Then, they exposed the iPS cells to proteins—known as growth factors and cytokines—suspected to be key to airway development. To see whether any given mixture of proteins was effective, the researchers then tested whether the resulting tissue had each type of airway cell arranged in the right way and contained hair-like cilia and key molecules needed for airway function. After trial and error, they found the precise mix of growth factors needed to coax the IPS cells into fully functional airway tissue.
"The ability to generate a variety of cells that compose a fully mature lung is the first step to understand the molecular mechanisms of many lung diseases," says Verma, who is also an American Cancer Society Professor of Molecular Biology.
While the grown cells aren't identical to those found in all parts of the lung, similar procedures could pin down how to grow tissue from other sections of the respiratory system. Already, the new protocol can be used to develop cell populations to study rare diseases, such as primary ciliary dyskinesia, known to affect the cilia lining airways. In addition, new treatments or genetic therapies could be tested on lab-grown airway tissue derived from an affected patient's cells.
"It will hopefully become possible one day to correct the genetic mutations in these tissues and engraft them back into the airways of a patient," says Firth.
Airway tissue grown in the lab could also reveal how pollutants or nicotine exacerbate symptoms of diseases not only affected by genetics but by the air a person breathes.
The research was a collaboration with Fred Gage, Salk professor of genetics and holder of the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease. Other researchers on the study were Carl Dargitz, Susan Qualls, Tushar Menon, Rebecca Wright, Oded Singer, and Ajai Khanna of the University of California, San Diego.
The work was supported by the Berger Foundation and a California Institute of Regenerative Medicine grant, and made use of facilities and equipment supported by the Waitt Foundation, National Cancer Institute, National Institute of Neurological Disorders and Stroke, California Institute of Regenerative Medicine, and Leona M. and Harry B. Helmsley Charitable Trust.
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world's preeminent basic research institutions, where internationally renowned faculty probes fundamental life science questions in a unique, collaborative and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer's, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent nonprofit organization and architectural landmark.
JOURNAL
Proceedings of the National Academy of Sciences
AUTHORS
Amy L. Firth, Carl T. Dargitz, Susan J. Qualls, Tushar Menon, Rebecca Wright, Oded Singer, Fred Gage, and Inder M. Verma of the Salk Institute for Biological Studies; Ajai Khanna of the University of California, San Diego
Office of Communications
Tel: (858) 453-4100
press@salk.edu