
August 12, 2021
Salk scientists show how blocking opioid receptors in specific neurons can restore breathing during an overdose
Salk scientists show how blocking opioid receptors in specific neurons can restore breathing during an overdose
LA JOLLA—It’s long been known that opioid overdose deaths are caused by disrupted breathing, but the actual mechanism by which these drugs suppress respiration was not understood. Now, a new study by Salk scientists has identified a group of neurons in the brainstem that plays a key role in this process.
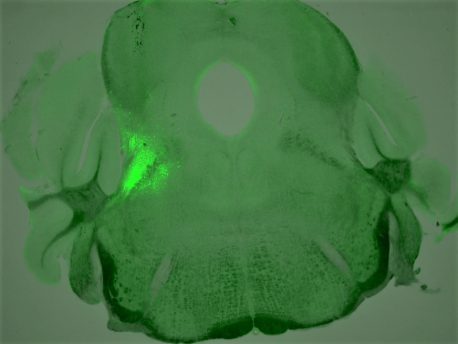
The findings, published June 8, 2021 in the journal Proceedings of the National Academy of Sciences, show how triggering specific receptors in these neurons causes opioid-induced respiratory depression, or OIRD, the disrupted breathing that causes overdose deaths. It also shows how blocking these receptors can cause OIRD to be reversed.
“The underlying mechanism of why opiates slow down and depress the breathing rhythm has not been fully characterized,” says senior investigator Sung Han, assistant professor in Salk’s Clayton Foundation Laboratories for Peptide Biology. “This knowledge can provide a stepping stone to better treatment options for OIRD.”
The U.S. saw more than 93,000 overdose deaths in 2020, of which an estimated 60 percent were attributed to opioids like fentanyl. Opioids work by binding to proteins on nerve cells (neurons) called opioid receptors and subsequently inhibiting their activity. Currently, naloxone is the only medication known to block the effects of opioids and reverse an overdose. But naloxone has limitations, including a short duration that requires it to be administered multiple times. It also works systemically, blocking opioid receptors throughout the entire body, including those that control pain.
To develop strategies to rescue OIRD with more specificity, Han’s team set out to search for the breathing neurons in the brain that also carry opioid receptors. In the new study, the researchers identified a group of neurons that express a certain type of opioid receptor (the mu opoid receptor) and are located in the brainstem breathing modulation center; they then characterized these neurons’ role in OIRD.
They found that mice that were genetically engineered to lack opioid receptors in these neurons didn’t have their breathing disrupted when exposed to morphine, as mice in the control group did. The researchers also found that, without introducing opioids, stimulating these receptors in control mice caused symptoms of OIRD.
The team then looked at ways to reverse the process by treating the overdosed mice with chemical compounds targeted to other receptors on the same neurons, which play an opposite role as the opioid receptor (activating rather than inhibiting them).
“We discovered four different chemical compounds that successfully activated these neurons and brought back the breathing rate during OIRD,” said first author Shijia Liu, a graduate student in the Han lab. Recovery in overdosed mice was close to 100 percent, which surprised the team.
The researchers next plan to look at whether other cell groups also play a role in OIRD. Further study would also examine the connection between breathing regulation and pain perception in the brain, potentially opening the door to developing more targeted treatments for OIRD.
“We hope to explain the pain-breathing segregation at the molecular or microcircuit level,” says Han, who holds the Pioneer Fund Development Chair. “By doing that, we can try to restore breathing without touching analgesic effects of opioids.”
Other authors on the study are Dong-Il Kim, Tae Gyu Oh, Gerald M. Pao, Jong-Hyun Kim, Kuo-Fen Lee and Ronald M. Evans of Salk; Richard Palmiter of the University of Washington; and Matthew R. Banghart of the University of California San Diego.
The research was supported by the National Institute of Mental Health, the Simons Foundation Autism Research Initiative, the Brain Research Foundation, the Klingenstein-Simons Fellowship Award in Neuroscience, and the Rita Allen Foundation.
DOI: 10.1073/pnas.2022134118
JOURNAL
Proceedings of the National Academy of Sciences
AUTHORS
Shijia Liu, Dong-Il Kim, Tae Gyu Oh, Gerald M. Pao, Jong-Hyun Kim, Richard D. Palmiter, Matthew R. Banghart, Kuo-Fen Lee, Ronald M. Evans, and Sung Han
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.