
May 11, 2023
Salk scientists establish organoid model for understanding how brain immune cells impact brain conditions, like autism spectrum disorder and Alzheimer’s disease
Salk scientists establish organoid model for understanding how brain immune cells impact brain conditions, like autism spectrum disorder and Alzheimer’s disease
LA JOLLA—Situated at the intersection of the human immune system and the brain are microglia, specialized brain immune cells that play a crucial role in development and disease. Although the importance of microglia is undisputed, modeling and studying them has remained a difficult task.
Unlike some human cells that can be studied outside of the body or in nonhuman models, human microglia are difficult to study when removed from the human-brain-like environment. To overcome this barrier, Salk scientists developed an organoid model—a three-dimensional collection of cells that mimics features of human tissues. This model allows researchers to study human microglial development and function for the first time in living human-derived tissue. Further, the scientists examined patient-derived microglia from children with macrocephalic autism spectrum disorder (a condition where infant head circumference is greater than 97 percent of other infants’) to determine whether brain environment influences the development of more reactive microglia.
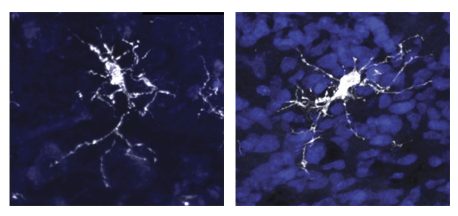
The findings, published in Cell on May 11, 2023, highlight the importance of immune cell and brain interaction, and improve the understanding of neurodegenerative and developmental diseases, such as autism spectrum disorder and Alzheimer’s disease.
“Outside of the brain environment, microglia lose almost all function and meaning,” says Professor Rusty Gage, senior author and holder of the Vi and John Alder Chair for Research on Age-Related Neurodegenerative Disease. “We knew that if we found a way to replicate the human brain environment in an organoid in order to study human microglia, then we would finally have a tool for examining how the heathy and diseased brain influence microglia and, reciprocally, how healthy and diseased microglia influence the brain.”
Emerging roughly 10 years ago, organoids have become a prevalent tool to bridge the gap between cell and human studies. Organoids can mimic human development and organ generation better than other laboratory systems, allowing researchers to study how drugs or diseases affect human cells in a more realistic setting. Brain organoids are typically grown in culture dishes, but the organoids are structurally and functionally limited by the lack of blood vessels, short survival time, and inability to sustain diverse cell types (like microglia).

“To create a brain organoid model that contains mature microglia and enables us to research them, we used a novel transplantation technique to create a human-brain-like environment” says co-first author Abed Mansour, a former postdoctoral researcher in Gage’s lab and now an assistant professor at the Hebrew University of Jerusalem. “So we could finally make a human brain organoid that had all the features necessary to orchestrate human microglia growth, behavior, and function.”
Unlike previous models, the researchers created a human brain organoid that had microglia and a human-brain-like environment, which finally allowed them to look at environmental influences on microglia throughout brain development. They found that a characteristic protein called SALL1 appeared as early as eleven weeks into development and served to confirm microglial identity and promote mature function. Additionally, they found that brain environment-specific factors, like the proteins TMEM119 and P2RY12, were necessary for microglia to function.
“Creating a human brain model that can effectively replicate the human brain environment is very exciting,” says Associate Professor Axel Nimmerjahn, another author of the study. “With this model, we can finally investigate how human microglia function within the human brain environment.”

As the team learned more about microglia, the importance of the relationship between brain environment and microglia became clear—especially in disease scenarios. The lab previously examined neurons derived from people with autism spectrum disorder and found their neurons grew faster and had more complex branches than neurotypical counterparts. With the new organoid model, the team could ask whether those neuronal differences altered the brain environment and influenced microglia development.
To do so, they compared microglia derived from skin samples from three individuals with macrocephalic autism spectrum disorder versus three neurotypical individuals with macrocephaly. The researchers found that individuals with autism spectrum disorder exhibited the neuronal differences the team had previously noted, and that the microglia were influenced by those differences in their growth environment. Because of this neuron-dependent environmental change, the microglia became more reactive to damage or intruders—a finding that may explain the brain inflammation observed in some individuals with autism spectrum disorder.
Since this was a preliminary study with a small sample size, the team plans to examine more microglia from additional people in the future to verify their findings. They also aim to expand their research to study other developmental and neurodegenerative diseases to see how microglia are contributing to disease onset.
“Rather than deconstruct the brain, we decided to construct it ourselves,” says co-first author Simon Schafer, a former postdoctoral researcher in Gage’s lab and now an assistant professor at Technical University of Munich. “By building our own brain model we can work from the bottom up and see solutions that may be impossible to see from the top down. We are eager to continue improving on our model and unravelling the relationship between the brain and immune system.”
Other authors include Monique Pena, Saeed Ghassemzadeh, Lisa Mitchell, Amanda Mar, Daphne Quang, Sarah Stumpf, and Clara Baek of the Salk Institute; Johannes C. M. Schlachetzki, Addison J. Lana, and Christopher K. Glass of UC San Diego; Irene Santisteban of the Technical University of Munich; and Raghad Zaghal of the Hebrew University of Jerusalem.
The work was supported by the National Institutes of Health (R01 AG056306, R01 AG057706, R01 AG056511, R01 AG061060, R01 NS108034, U19 NS123719, NCI CCSG: P30 014195, NCI CCSG: P30 014195), the American Heart Association and Paul G. Allen Frontiers Group (grant 19PABHI34610000), the Brain and Behavior Research Foundation (27685 and 30421), the German Research Foundation (500300695), the Milky Way Research Foundation, Annette C. Merle-Smith and the Robert and Mary Jane Engman Foundation, the European Molecular Biology Organization (ALTF 1214-2014), the Human Frontier Science Program (LT001074/2015), the European Research Council, the Chapman Foundation, the JBP Foundation and the Helmsley Charitable Trust.
DOI: 10.1016/j.cell.2023.04.022
JOURNAL
Cell
AUTHORS
Simon T. Schafer, Abed A. Mansour, Johannes C.M. Schlachetzki, Monique Pena, Saeed Ghassemzadeh, Lisa Mitchell, Amanda Mar, Daphne Quang, Sarah Stumpf, Irene Santisteban Ortiz, Addison J. Lana, Clara Baek, Raghad Zaghal, Christopher K. Glass, Axel Nimmerjahn, Fred H. Gage
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.