Associate Professor
Laboratory of Genetics
Hearst Foundation Developmental Chair


Biological life is organized along a continuum that ranges from complete living organisms down to tissues, cells, large “macromolecular” assemblies composed of proteins and nucleic acids, small “molecular” assemblies or individual molecules, and, finally, atoms. Since the advent of light microscopy several centuries ago, researchers have been unraveling the connection between biological structure and function along this continuum at increasingly finer degrees of spatial resolution. As technology improves, many researchers are finding that directly visualizing the structure of individual macromolecules or their assemblies at resolutions nearing the level of individual atoms can better reveal various types of dysfunction that lead to disease.
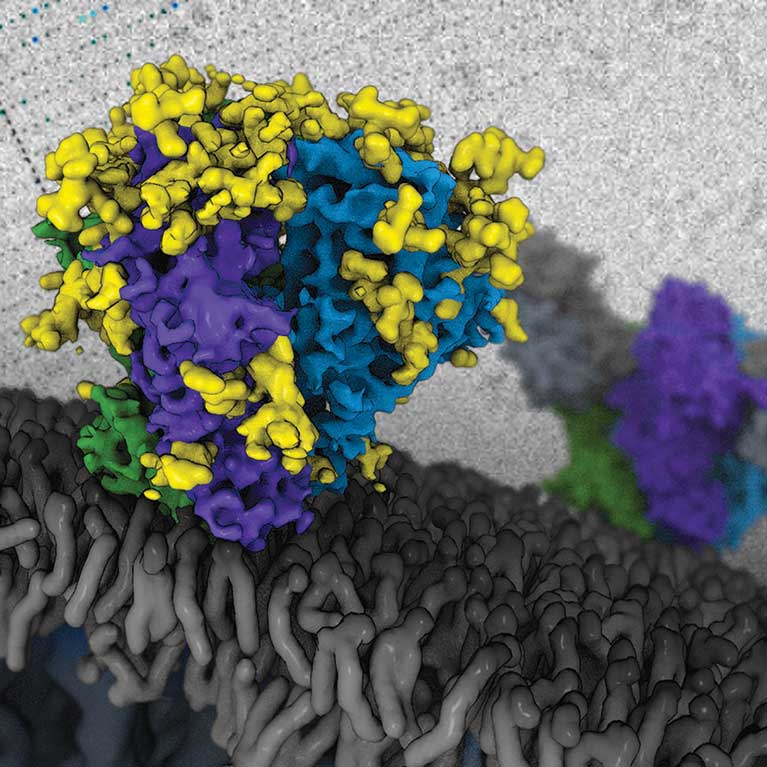
Dmitry Lyumkis utilizes and develops cutting-edge transmission cryo-electron microscopy (cryo-EM) techniques to determine the structures of macromolecules and macromolecular assemblies, which perform most of the functions inside cells. By observing previously unseen structures under different physiological conditions and at near-atomic resolution, Lyumkis aims to understand and interconnect the complex roles macromolecules play in human diseases such as cancer and HIV.
Lyumkis determined structures of macromolecular assemblies called “intasomes” from viruses including and related to HIV, which allows them to establish permanent infection in target host cells. These structures further our understanding of the molecular hallmarks of infection and, importantly, provide direct chemical blueprints for improving antiviral therapies used to treat HIV-infected individuals.
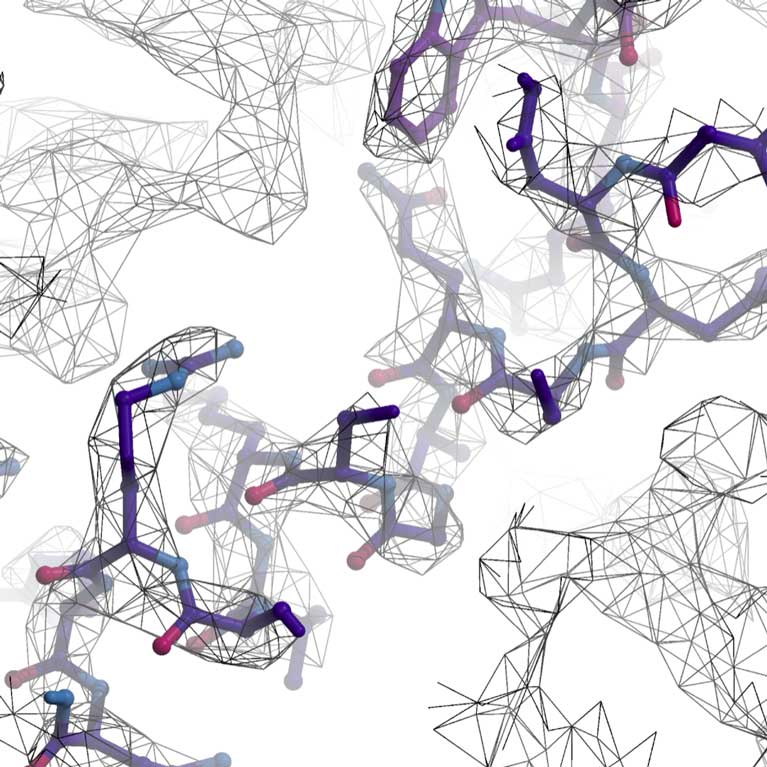
Lyumkis developed new methods to quantitatively evaluate and experimentally improve anisotropic (directionally dependent) resolution in cryo-EM, which frequently plagues attempts to derive meaningful structural information from biological samples. The techniques were shown to yield higher quality data and have broad applicability to structure determination and evaluation.

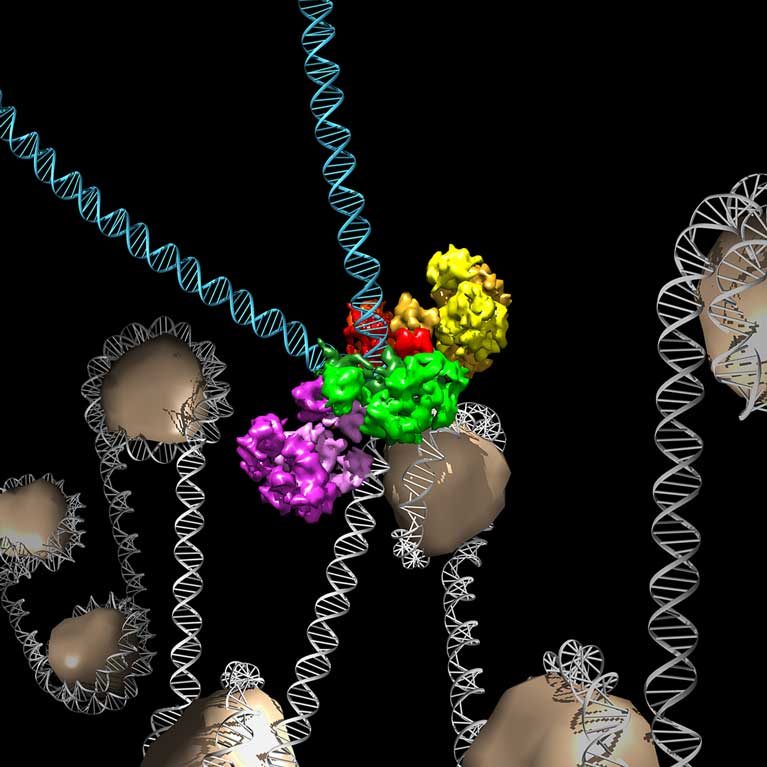
Lyumkis and colleagues deciphered the structures and molecular mechanisms of activity of a novel class of CRISPR/Cas enzymes, which has the ability to cut and edit RNA. This work opens novel opportunities for genetic engineering and has broad implications for understanding, and potentially treating, diseases at a molecular level.
BS, University of California San Diego
PhD, The Scripps Research Institute