
May 5, 2022
Salk scientists defined the molecular movement that connects gut to brain to behavior in worm models—a mechanism that may also occur in humans
Salk scientists defined the molecular movement that connects gut to brain to behavior in worm models—a mechanism that may also occur in humans
LA JOLLA–Whether it’s making rash decisions or feeling grumpy, hunger can make us think and act differently—“hangry,” even. But little is known about how hunger signals in the gut communicate with the brain to change behavior. Now, Salk scientists are using worms as a model to examine the molecular underpinnings and help explain how hunger makes an organism sacrifice comfort and make risky decisions to get a meal.
Their latest findings, published in PLOS Genetics on May 5, 2022, reveal that proteins in intestinal cells move dynamically to transmit signals about hunger, ultimately driving worms to cross toxic barriers to reach food. Similar mechanisms may also occur in humans.
“Animals, whether it’s a humble worm or a complex human, all make choices to feed themselves to survive. The sub-cellular movement of molecules could be driving these decisions and is maybe fundamental to all animal species,” says senior author Sreekanth Chalasani, associate professor in Salk’s Molecular Neurobiology Laboratory.
Chalasani and team used a tiny worm called Caenorhabditis elegans as a model to determine how hunger leads to behavioral changes. The researchers created a barrier of copper sulfate, which is a known worm repellant, between the hungry worms and a food source. They observed that if the worms were deprived of food for two-to-three hours, then they were more willing to traverse the toxic barrier compared to well-fed worms.
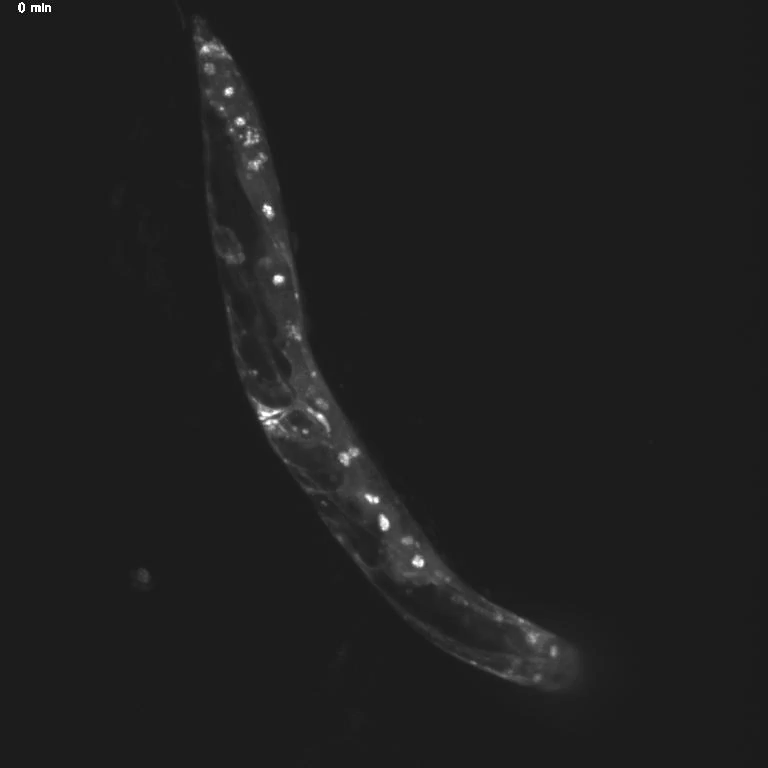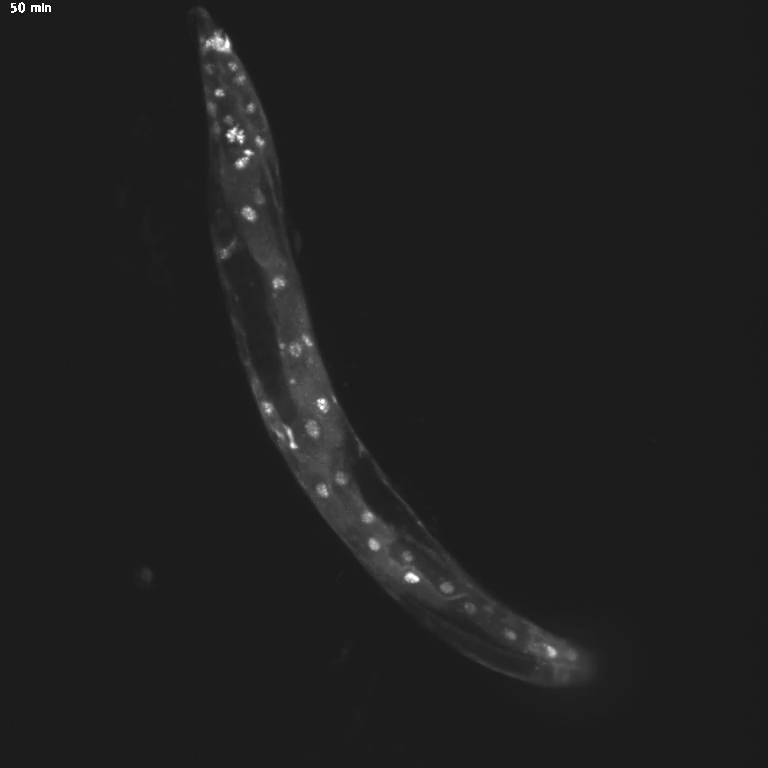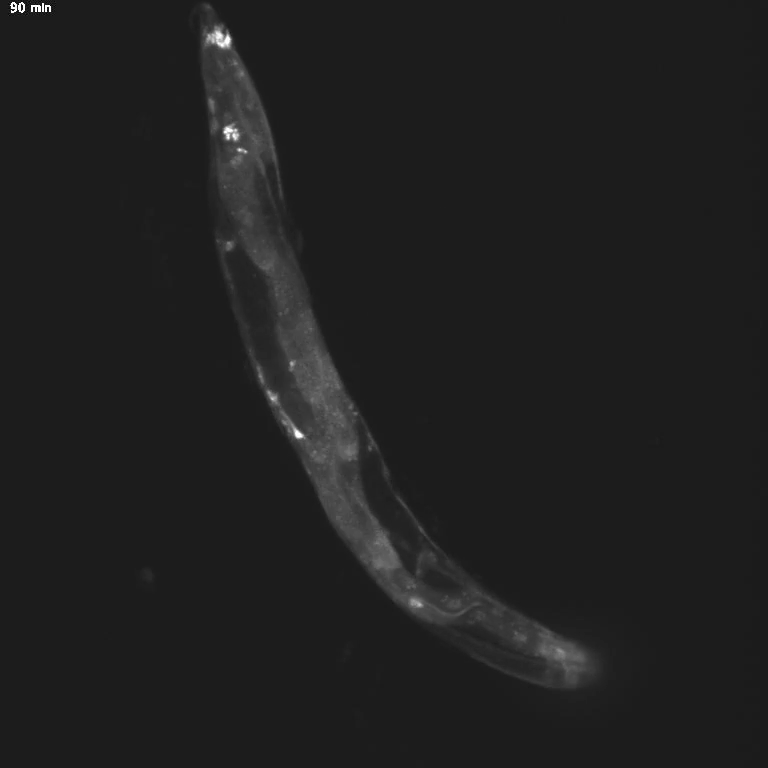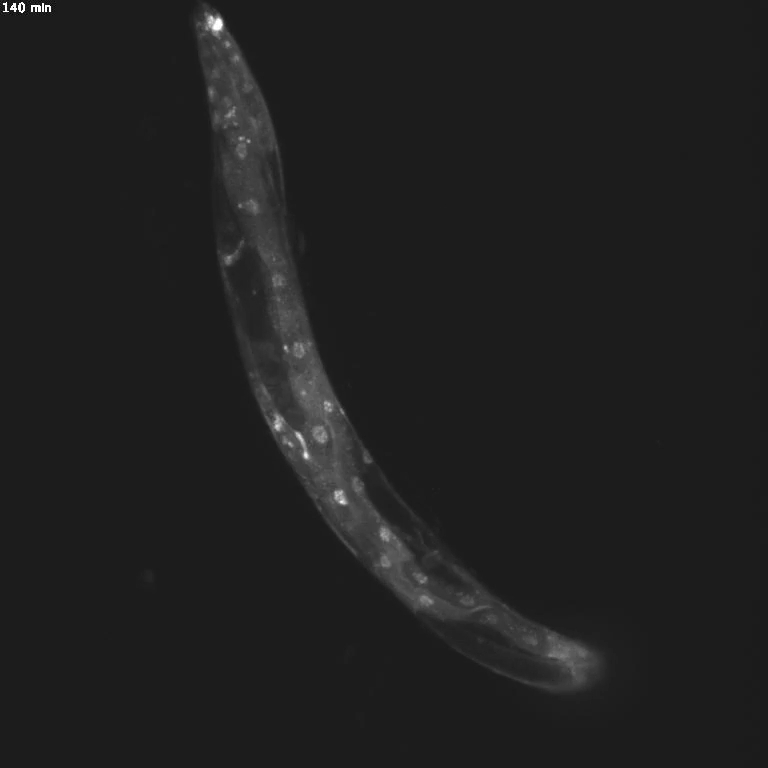
Using genetic tools and imaging techniques, the researchers then investigated the gut molecules that might be sending signals to the brain. They found that specific transcription factors, proteins that turn genes “on” and “off,” shifted locations in hungry animals. Normally, transcription factors hang out in the cell’s cytoplasm and move into the nucleus only when activated—similar to the way we live at home but go into the office to get work done.
The team was surprised to discover that these transcription factors, called MML-1 and HLH-30, move back to the cytoplasm when the worm is hungry. When the scientists deleted these transcription factors, hungry worms stopped trying to cross the toxic barrier. This indicates a central role for MML-1 and HLH-30 in controlling how hunger changes animal behavior.
In a follow-up experiment, the researchers also discovered that a protein called insulin-like peptide INS-31 is secreted from the gut when MML-1 and HLH-30 are on the move. Neurons in the brain, in turn, make a receptor that might detect the INS-31 secretions.

To sum it up: A lack of food leads to movement of MML-1 and HLH-30, which could promote the secretion of INS-31. INS-31 peptides then bind receptors on neurons to relay hunger information and drive risky food-seeking behavior.
“C. elegans are more sophisticated than we give them credit for,” says co-first author Molly Matty, a postdoctoral fellow in Chalasani’s lab. “Their intestines sense a lack of food and report this to the brain. We believe these transcription factor movements are what guide the animal into making a risk-reward decision, like traversing an unpleasant barrier to get to food.”
Next, the scientists will further investigate the dynamic nature of these transcription factors and underlying mechanisms. With further work, these findings could provide insight into how other animals, such as humans, prioritize basic needs over comfort.
This work was supported by the Rita Allen Foundation, W.M. Keck Foundation, National Institutes of Health (grant R01MH096881), National Science Foundation (postdoctoral research fellowship 2011023 and two graduate research fellowships), Glenn Foundation and Socrates Program (grant NSF-742551).
Other authors included Hiu Lau, Jessica Haley, Anupama Singh, Ahana Chakraborty, Karina Kono and Kirthi Reddy of Salk; and Malene Hansen of Sanford Burnham Prebys.
JOURNAL
PLOS Genetics
TITLE
Intestine-to-neuronal signaling alters risk-taking behaviors in food-deprived Caenorhabditis elegans
AUTHORS
Molly A. Matty, Hiu E. Lau, Jessica A. Haley, Anupama Singh, Ahana Chakraborty, Karina Kono, Kirthi C. Reddy, Malene Hansen, and Sreekanth H. Chalasani
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.