
September 19, 2016
Salk Institute researchers and collaborators develop novel cancer treatment that halts fat synthesis in cells, stunting tumors
Salk Institute researchers and collaborators develop novel cancer treatment that halts fat synthesis in cells, stunting tumors
LA JOLLA—Fat isn’t just something we eat: it may also lie at the heart of a new approach to treating cancer.
Cells create their own fat molecules to build their plasma membranes and other critical structures. Now, researchers at the Salk Institute, along with academic and industry collaborators, have found a way to obstruct this instrumental process to stifle cancer’s growth, detailed September 19, 2016 in Nature Medicine. Like halting the delivery of supplies to a construction site, the approach stalls the molecular building blocks cancer needs to grow.
“Cancer cells rewire their metabolism to support their rapid division,” says Salk Professor Reuben Shaw, whose lab has made significant progress in establishing the ties between cancer and metabolic processes. “Because cancer cells are more reliant on lipid synthesis activity than normal cells, we thought there might be subsets of cancers sensitive to a drug that could interrupt this vital metabolic process.”
Researchers had previously hypothesized that interrupting cells’ lipid assembly line could disable cancer, but it was only recently that they were able to disrupt the process and test this theory. Shaw’s team partnered with a Boston-based biotech, Nimbus Therapeutics, which discovers and develops small molecules in the hopes of treating a variety of diseases, who were developing a molecule to shut off a critical player in lipid synthesis, an enzyme called acetyl-CoA carboxylase, or ACC.
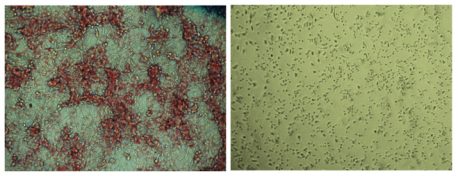
Click here for a high-resolution image.
Credit: Salk Institute
“This confirms that shutting down endogenous lipid synthesis could be beneficial in some cancers and that inhibitors of the ACC enzyme represent a feasible way to do it,” said Rosana Kapeller, Chief Scientific Officer at Nimbus Therapeutics and a co-author of the paper. “We’ve taken a novel computational chemistry approach to designing high-potency allosteric inhibitors of this difficult enzyme, and we are very encouraged by the results.”
In multiple and extensive large-scale tests in both animal models of cancer and in transplanted human lung cancer cells, the results of the novel ACC inhibitor, dubbed ND-646, were far more promising than expected: tumor mass shrank by roughly two-thirds compared to untreated animals. And when the researchers paired ND-646 with one of the common treatments for non-small lung cancer called carboplatin, the anti-tumor response was even greater: a dramatic 87 percent of tumors were suppressed, compared to 50 percent with the standard treatment of carboplatin alone.
This combination of carboplatin (which damages DNA, a problem for rapidly dividing cells) and ND-646 (knocking out ACC and halting lipid synthesis) didn’t seem to impair normal cells even as it dramatically slowed cancer growth.
“We found surprisingly well-tolerated dosing with some of these novel ACC inhibitors that have broad bioavailability and should not be far away from what would be needed to initiate clinical trials,” says first author Robert Svensson, a Salk research associate.

Click here for a high-resolution image.
Credit: Salk Institute
“This is the first time anyone has shown that this enzyme, ACC, is required for the growth of tumors and this represents compelling data validating the concept of being able to target fat synthesis as a novel anticancer approach,” adds Shaw, who is the holder of the William R. Brody Chair. “The implications are that we have a very promising drug for clinical trials for subtypes of lung cancer as well as liver and other types of cancer. This represents a new weapon in the arsenal to fight cancer.”
Other authors on the work include: Lillian J. Eichner, Matthew J. Kolar, Sonja N. Brun, Portia S. Lombardo, Jeanine L. Van Nostrand, Amanda Hutchins, Lilliana Vera, Laurie Gerken, and Alan Saghatelian of the Salk Institute; Jeremy Greenwood and Sathesh Bhat of Schrödinger; Geraldine Harriman, William F. Westlin and H. James Harwood Jr., of Nimbus Therapeutics; and Seth J. Parker, Martina Wallace, and Christian M. Metallo of the University of California, San Diego.
The work was funded by: National Institutes of Health (R01CA172229, P01CA120964), the Samuel Waxman Cancer Research Foundation, The Leona M. and Harry B. Helmsley Charitable Trust and the Department of Defense.
About ND-646: Gilead Sciences, Inc. acquired Nimbus’ ACC inhibitor program in May 2016, including ND-646 and other ACC inhibitors for the treatment of non-alcoholic steatohepatitis (NASH) and for the potential treatment of hepatocellular carcinoma (HCC) and other diseases. For more information, please visit www.NimbusTx.com.
JOURNAL
Nature Medicine
AUTHORS
Robert U. Svensson, Seth J. Parker, Lillian J. Eichner, Matthew J. Kolar, Martina Wallace, Sonja N. Brun, Portia S. Lombardo, Jeanine L. Van Nostrand, Amanda Hutchins, Lilliana Vera, Laurie Gerken, Jeremy Greenwood, Sathesh Bhat, Geraldine Harriman, William F. Westlin, H. James Harwood Jr., Alan Saghatelian, Rosana Kapeller, Christian M. Metallo and Reuben J. Shaw
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.