
January 30, 2020
During development, cells generated from people with autism have frequent breaks in the DNA of certain genes
During development, cells generated from people with autism have frequent breaks in the DNA of certain genes
LA JOLLA—Researchers at the Salk Institute have discovered a unique pattern of DNA damage that arises in brain cells derived from individuals with a macrocephalic form of autism spectrum disorder (ASD). The observation, published in the journal Cell Stem Cell, helps explain what might go awry in the brain during cell division and development to cause the disorder.
“Division, or replication, is one of the most dangerous things that a cell can do,” says Salk Professor Rusty Gage, the study’s senior author and president of the Institute. “Most DNA damage is repaired through a remarkably efficient repair process, but errors occur when the rate of division is altered genetically or environmentally, which can lead to long-term functional defects.”
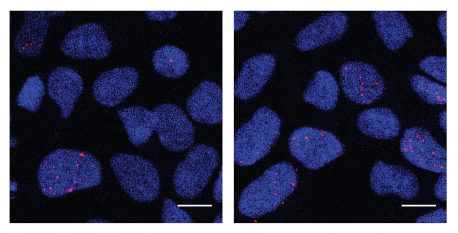
Click here for a high-resolution image.
Credit: Salk Institute
ASD, a developmental disorder of communication and behavior, affects about 1 in 59 children in the US, according to the Centers for Disease Control and Prevention. Research into the underlying causes of the disorder, as well as possible treatments, has been slow.
In 2016, Gage and his colleagues discovered that brain stem cells from people with the macrocephalic form of autism grew more quickly than cells from unaffected individuals. (Brain stem cells are precursors to more-specialized types of cells, such as neurons.) The finding explained, in part, why many people with ASD also have macrocephaly, or unusually large heads: more proliferation of brain stem cells during development can lead to larger brains.
In the new research, Gage and his colleagues again looked at these neural precursor cells (NPCs). As all cell types proliferate and mature during embryonic development, it’s normal for their quickly-replicating strands of DNA to accumulate small errors, most of which are corrected and never do any harm. The researchers wondered whether this DNA damage that occurred during the stress of replication was more common in the quickly-dividing neural precursors of people with autism.
The researchers collected skin cells from individuals with both ASD and macrocephaly, as well as from neurotypical individuals (without ASD), and used stem-cell reprogramming technology to coax each person’s cells into NPCs.
Gage’s team used a chemical compound to induce replication stress on the NPCs derived from people without autism and studied where DNA damage was most likely to accumulate. They compared this induced damage in cells from individuals without autism to where DNA damage naturally accumulated in the cells from people with autism. The NPCs from autistic individuals had heightened levels of DNA damage, clustered in 36 of the same genes that had also been damaged in healthy cells exposed to replication stress. And 20 of the genes had been previously linked to autism in separate genetic studies.

Click here for a high-resolution image.
Credit: Salk Institute
“What the new results are telling us is that cells from people with macrocephalic autism not only proliferate more, but also naturally experience more replication stress,” says Meiyan Wang, a graduate student in the Gage lab and first author of the new paper.
Fast proliferation of NPCs may lead to both macrocephaly and cellular stress that spurs DNA damage, she says. That damage could be one source of mutations associated with ASD. While the technology used in the study told the researchers where DNA damage occurred, they don’t know how much of that damage was repaired before cells matured into adult neurons and how much of it leads to lasting mutations.
“We’d like to look deeper at how replication stress and DNA damage affects neuronal function in the long term and whether adult neurons arising from these stem cells have more mutations than usual,” says Wang.
Other researchers on the paper were Christina Lim, Iryna Gallina, Sara Marshall and Maria Marchetto of Salk and Pei-Chi Wei and Frederick Alt of Boston Children’s Hospital.
The work and researchers involved were supported by grants from the California Institute for Regenerative Medicine, an AHA-Allen Initiative in Brain Health and Cognitive Impairment award made jointly through the American Heart Association and The Paul G. Allen Frontiers Group, the National Institutes of Health, the JPB Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, Annette C. Merle-Smith, the Robert and Mary Jane Engmann Foundation, the March of Dimes, the Harvard Brain Initiative, the Charles H. Hood Foundation, the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, and the Howard Hughes Medical Institute.
DOI: 10.1016/j.stem.2019.12.013
JOURNAL
Cell Stem Cell
AUTHORS
Meiyan Wang, Pei-Chi Wei, Christina K. Lim, Iryna S. Gallina, Sara Marshall, Maria C. Marchetto, Frederick W. Alt and Fred H. Gage
JOURNAL COVER

COVER: In this issue, Wang et al. (221–233) find that in neural progenitors derived from autism patients, increased proliferation induces replication‐stress‐associated genome instability, which leads to increased collisions of the replication fork and transcription machineries. The illustration depicts the collisions of replication and transcription represented as two spaceships. The contrail traces the path of the spaceship, in reference to the genomic DNA on which both transcription and replication operate. Cover artwork by Amy Cao.
Office of Communications
Tel: (858) 453-4100
press@salk.edu
The Salk Institute is an independent, nonprofit research institute founded in 1960 by Jonas Salk, developer of the first safe and effective polio vaccine. The Institute’s mission is to drive foundational, collaborative, risk-taking research that addresses society’s most pressing challenges, including cancer, Alzheimer’s, and agricultural resilience. This foundational science underpins all translational efforts, generating insights that enable new medicines and innovations worldwide.