
November 21, 2016
Salk Institute finding could lead to treatment designs for immune-based cancer and autoimmune disease
Salk Institute finding could lead to treatment designs for immune-based cancer and autoimmune disease
Click here for a high-resolution image.
Credit: Salk Institute
LA JOLLA—When a receptor on the surface of a T cell—a sentry of the human immune system—senses a single particle from a harmful intruder, it immediately kicks the cell into action, launching a larger immune response. But exactly how the signal from a single receptor, among thousands on each T cell, can be amplified to affect a whole cell has puzzled immunologists for decades.
Now, Salk scientists have discovered the key to the amplification of an “invader” signal. The T cell receptor that detects the intruder turns into a mini-machine, activating and releasing copy after copy of a protein called ZAP70. The finding, published in Nature Immunology on November 21, 2016, could help scientists design better immune-mediated treatments for cancer or autoimmune diseases.
“This is really the first amplification method that’s been found at this level of the immune response,” says senior author Björn Lillemeier, an associate professor in Salk’s Nomis Foundation Laboratories for Immunobiology and Microbial Pathogenesis and the Waitt Advanced Biophotonics Center. “It answers a longstanding question that has bugged immunologists for more than three decades.”
T cells are central in the adaptive immune response, which is the body’s ability to recognize pathogens and respond to them. A single T cell’s receptors screen thousands of molecules at any given second, but most of them originate from the body’s own proteins and have to be ignored as “self.” Researchers have struggled to explain how, in the wake of overwhelming “self” signals, a T cell can detect and respond to one or two “invader” signals.
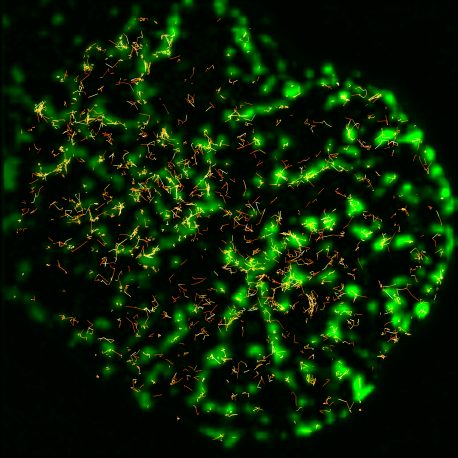
Lillemeier’s lab studied ZAP70, a protein that associates with T cell receptors and becomes activated when the receptors recognize a foreign molecule. To track the activity and location of ZAP70 molecules, the team tagged them with a fluorescent marker while anchoring each T cell receptor in place. To the group’s surprise, ZAP70 molecules were being activated by the T cell receptors and then moving away, spreading throughout the cell.
“This is a completely different method of amplification than we expected,” says Zachary Katz, a research associate in the Lillemeier lab and first author of the new work. “Everyone always thought the amplification would be determined by the interaction between the foreign molecule and the T cell receptor, but this is happening downstream of the receptor.”

Click here for a high resolution image
Credit: Salk Institute
By churning out ZAP70 and sending it throughout the cell—as opposed to just activating a handful of ZAP70s and keeping them tethered to the T cell receptor—the immune cells can rapidly spread a signal throughout the cell.
“What we saw is that at the beginning of signaling, you have lots of ZAP70 being released from the T cell receptor to amplify and distribute the signal,” says Lillemeier. “But once the signaling is established, the T cell receptor actually adapts and stops releasing so much of ZAP70.”
Questions remain on how the process works, including what the ultimate destinations of the ZAP70 molecules are and how they go on to transmit signals. But the observation, Lillemeier says, is progress toward understanding how T cells identify and react to pathogens.
“It’s really important to understand this process since T cells are at the center of the adaptive immune response,” he says. “If the receptors are not controlled well, you’re sick; you might either have an autoimmune disease or you can’t respond to infections.” Being able to make the receptors have a stronger or weaker signal—perhaps by changing how much ZAP70 they activate and release—could help treat these kinds of diseases, he adds.
Other researchers on the study were Lucie Novotná and Amy Blount of the Salk Institute.
The work and the researchers involved were supported by grants from the Nomis Foundation, the Waitt Foundation and the James B. Pendleton Charitable Trust, the National Institutes of Health, a Pioneer Fund Postdoctoral Scholar Award, the Salk Institute Cancer Center core facilities funded by the National Cancer Institute and the Mass Spectrometry Core of the Salk Institute supported by the Helmsley Center for Genomic Medicine.
JOURNAL
Nature Immunology
AUTHORS
Zachary B Katz, Lucie Novotná, Amy Blount, Björn F Lillemeier
Office of Communications
Tel: (858) 453-4100
press@salk.edu
The Salk Institute is an independent, nonprofit research institute founded in 1960 by Jonas Salk, developer of the first safe and effective polio vaccine. The Institute’s mission is to drive foundational, collaborative, risk-taking research that addresses society’s most pressing challenges, including cancer, Alzheimer’s, and agricultural resilience. This foundational science underpins all translational efforts, generating insights that enable new medicines and innovations worldwide.