
May 10, 2023
Salk scientists investigate how neuropeptides affect specific neurons, providing insight into how the brain communicates in autism-spectrum and attention-deficit disorders
Salk scientists investigate how neuropeptides affect specific neurons, providing insight into how the brain communicates in autism-spectrum and attention-deficit disorders
LA JOLLA—In addition to communicating with neurotransmitters, the brain also uses small proteins called neuropeptides. Neuropeptides send signals between neurons, working similarly to neurotransmitters but with key differences like a greater size and an ability to travel far away from the neuron that produces them. Though their importance is widely recognized, the way neuropeptides move around the brain and influence neurons has remained poorly understood—until now.
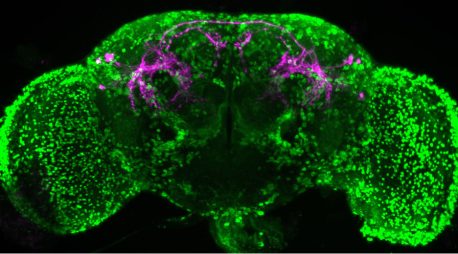
A study by Salk scientists published in the Journal of Neuroscience on May 10, 2023, reveals the variable influence that neuropeptides can have on brain activity and illuminates how the brain communicates in flies—an important step to understanding the underlying causes of human conditions, like autism-spectrum disorder or attention-deficit disorders.
“Studies have been treating neuropeptides like they have a single origin and single target that produces a simple outcome, but they have far more influence than that,” says senior author Kenta Asahina, an associate professor in the Molecular Neurobiology Laboratory. “Neuropeptides can do many things, with diverse and previously unexplored effects that have huge impacts on behavior.”
Neuropeptides can affect a range of behaviors, including eating, mating, and sleeping, as well as feeling fearful or stressed. Some neurons release neuropeptides as signaling molecules, while other neurons receive neuropeptides via receptors located on the cell’s surface. Prior to the Salk study, neuropeptides were assumed to have a general, systemic effect on all neurons that express the matching receptor. But growing evidence suggests a particular neuropeptide might control different behaviors, like eating or being aggressive, by acting on distinct brain circuits.
In this study, Asahina’s team wanted to examine the neuropeptide tachykinin, which is known to increase aggression in many animal species, including fruit flies, mice, and even humans. They were curious to know how tachykinin affects neuron communication and animal behavior.
To track tachykinin, the team created a molecular flag to label fruit fly neurons with receptors for tachykinin. Using the flag, the team could visualize how tachykinin activated some neurons, which could create specific behaviors in the flies. The differences in expressed receptors, coupled with certain behaviors, represent a novel mechanism for neuronal communication using neuropeptides.
The differences in expressed receptors represent an often-overlooked mechanism for neuronal communication using neuropeptides, because one type of neuropeptides can activate different receptors at different concentrations.
Neurons trigger one another to fire like a line of falling dominoes, and neuropeptides help the process along—almost like giving each domino a little extra push. The researchers found that tachykinin from a single male-specific type of neuron (flies have sex-specific neurons) affected two separate downstream groups of neurons, which—like all dominoes after the first—depended on the neuropeptide signal to act. The first group of neurons expressed a specific tachykinin receptor (TkR86C) and were required for promoting aggressive behavior. The second group of neurons expressed another type of tachykinin receptor (TkR99D) and was only activated when tachykinin was overproduced. Tachykinin affected neurons in different parts of the brain and the neurons responded differently with varying tachykinin concentration. Additionally, the activity patterns in the two groups of neurons were related to the levels of male aggression in the fruit flies.

“By imaging fruit fly brains, we were able to see two distinct subsets of neurons that had receptors for the neuropeptide tachykinin,” says Margot Wohl, the paper’s first author and a former graduate student researcher in Asahina’s lab. “It turns out tachykinin was signaling to each receptor in different areas of the brain, but the receptors responded differently when we increased tachykinin concentration. This is likely a way to control complex behavior—like how the fly decides who to fight and with how much aggression—and can help us better understand the influence of neuropeptides across the brain.”
The findings highlight how neuropeptides released from a small group of neurons can reshape activity patterns in multiple downstream groups of neurons throughout the fly brain to impact behavior. This discovery also lays the foundation for future investigations into how neuropeptides impact complex behaviors.
“We may be looking at fruit flies, but the implications of our research extend far beyond them,” says Asahina. “We know that neuropeptides are also in human brains, and by looking at this comparatively simple fruit fly model we can begin to understand how neuropeptides may influence our own brains—in health and in dysfunction.”
Other authors include Jett Liu of the Salk Institute.
The work was supported by the Mary K. Chapman Foundation, Rose Hills Foundation, and National Institutes of Health (NIDCD R01 DC015577).
DOI: 10.1523/JNEUROSCI.1734-22.2023
JOURNAL
The Journal of Neuroscience
AUTHORS
Margot P. Wohl, Jett Liu, Kenta Asahina
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.