
July 14, 2016
Salk researchers and collaborators provide new benchmark for generating the most primitive type of stem cell
Salk researchers and collaborators provide new benchmark for generating the most primitive type of stem cell
LA JOLLA—Salk scientists and colleagues have proposed new molecular criteria for judging just how close any line of laboratory-generated stem cells comes to mimicking embryonic cells seen in the very earliest stages of human development, known as naïve stem cells. The tests found that no current protocols lead to truly naïve stem cells, but the guidelines may help researchers achieve that goal by pointing out where each current method falls short. Generating naïve stem cells would be a boon to both basic research and to medical applications of stem cells, such as growing tissue for organ replacement.
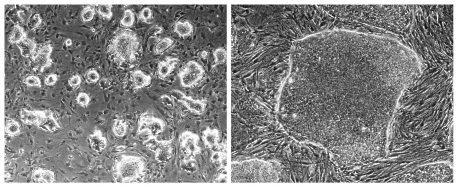
Click here for a high-resolution image
Credit: Salk Institute
“The naïve state potentially has a greater ability to generate different tissue types and could have many uses for regenerative medicine,” says senior author Joseph Ecker, professor and director of Salk’s Genomic Analysis Laboratory and a Howard Hughes Medical Institute investigator. The work was published online July 14, 2016 in Cell Stem Cell.
While stem cells—cells that have the potential to differentiate into other types of cells—exist in adult humans, the most useful stem cells are those found in embryos, which are pluripotent, capable of becoming nearly any cell in the body. Researchers have developed cocktails of molecules that turn back the clock on adult cells to make them act as stem cells (called induced pluripotent stem cells or iPSCs), and also have cultured lines of stem cells derived directly from embryos (ESCs). New methods are being formulated to coax the “primed” ESCs—which more resemble cells from post-implantation embryos—back in time even more to resemble naïve stem cells, those found in pre-implantation embryos only days after fertilization. Naïve stem cells are blank slates that form the basis for not only all the cells of the human body, but cells that make up the placenta to support an embryo as well.
“In our opinion, most of the published protocols to generate so-called naïve stem cells are not convincing because they produce cells that are very much like the starting cells—there’s not much difference in gene expression,” says co-senior author Rudolf Jaenisch of the Whitehead Institute for Biomedical Research and Massachusetts Institute of Technology.
Ecker, working closely with Jaenisch and other collaborators at Whitehead and the Ecole Polytechnique Federale de Lausanne, wanted to see whether these new techniques aiming to induce the naïve stem cell state truly did that. They performed a series of molecular tests on the “primed” cells and ESCs that had been exposed to factors thought to induce naïvety. They estimated the states of the two ESCs by comparing their molecular property and the cells from various stages in embryonic development from previous studies.
Three main tests, they found, were most indicative of the difference between naïve stem cells and other stem cells, letting them place each line of ESCs correctly along this timeline. First, they measured the expression levels of transposons, DNA sequences that can jump around the genome. The expression of certain transposons, they discovered, was indicative of naïve stem cells. Next, they found that the genomes of naïve embryonic stem cells have less methylation—the addition of methyl chemical groups along DNA. They then studied the state of X chromosomes in naïve cells of female embryos’, which each contain two active X chromosomes, unlike more mature embryonic cells that have silenced one X.

Click here for a high-resolution image
Credit: Salk Institute
Together, the three tests include tens of thousands of genetic biomarkers to characterize the developmental state of stem cells, says co-senior author Didier Trono of the Ecole Polytechnique Federale de Lausanne. “This type of analysis is likely to become a gold standard for quality controlling stem cells, including induced pluripotent stem cells, whether they are to be used solely in research or envisioned for clinical applications,” he says.
When current methods for generating naïve stem cells in the lab were judged using the three tests, each fell short of mimicking the naïve embryonic cells in different ways. One new technique, for instance, led to cells that had two active X chromosomes but didn’t match the exact methylation patterns desired.
“This really was a comparison of existing methods, applying the same criteria to each method and seeing where each cell state is,” says Ecker. “Some of these cells ended up being in states earlier in development and some later in development.”
Thorold Theunissen, a postdoctoral fellow in Jaenisch’s lab and co-first author of the study, says “Our work provides a rigorous set of criteria for comparing naïve human stem cells to their counterparts in the early human embryo. Previous studies mainly relied on comparisons with mouse stem cells, which are highly divergent from human.”
The scientists hope that other research teams adopt their criteria to judge their own methods and cell lines. “The transposon and methylation profiles are pretty standard in terms of technique and protocol so it’s pretty easy for other labs to repeat the experiment on, for example, naïve cells from a newly developed method,” says Yupeng He, a graduate student in the Ecker lab who helped lead the work.
Other researchers on the study were Styliani Markoulaki, Haoyi Wang, Malkiel A. Cohen, Katherine J. Wert, Yanmei Huang, Jesse Drotar, and Tenzin Lungjangwa of the Whitehead Institute for Biomedical Research; Marc Friedli, Evarist Planet, Julien Pontis, Alexandra Iouranova, Michael Imbeault, and Julien Duc of the Ecole Polytechnique Federale de Lausanne; and Ryan C. O’Neil, Rosa Castanon, Zhuzhu Zhang, and Joseph R. Nery of the Salk Institute.
The work and the researchers were supported by grants from the Simons Foundation, National Institutes of Health, Swiss National Science Foundation, European Research Council, Howard Hughes Medical Institute, Gordon and Betty Moore Foundation, Mary K. Chapman Foundation, a Sir Henry Wellcome Postdoctoral Fellowship, a Foundation Bettencourt Award, the Association Pour La Recherche Sur Le Cancer, and the Fonds de la Recherche en Sante du Quebec.
JOURNAL
Cell Stem Cell
AUTHORS
Thorold W. Theunissen, Styliani Markoulaki, Haoyi Wang, Malkiel A. Cohen, Katherine J. Wert, Yanmei Huang, Jesse Drotar, Tenzin Lungjangwa, and Rudolf Jaenisch of the Whitehead Institute for Biomedical Research; Marc Friedli, Evarist Planet, Julien Pontis, Alexandra Iouranova, Michael Imbeault, Julien Duc, and Didier Trono of the Ecole Polytechnique Federale de Lausanne; Yupeng He, Ryan C. O’Neil, Rosa Castanon, Zhuzhu Zhang, Joseph R. Nery, and Joseph R. Ecker of the Salk Institute.
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.