
June 7, 2021
Salk research shows how to optimize the production of insulin-producing cells from stem cells
Salk research shows how to optimize the production of insulin-producing cells from stem cells
LA JOLLA—Type 1 diabetes, which arises when the pancreas doesn’t create enough insulin to control levels of glucose in the blood, is a disease that currently has no cure and is difficult for most patients to manage. Scientists at the Salk Institute are developing a promising approach for treating it: using stem cells to create insulin-producing cells (called beta cells) that could replace nonfunctional pancreatic cells.
In a study published on June 7, 2021, in the journal Nature Communications, the investigators reported that they have developed a new way to create beta cells that is much more efficient than previous methods. Additionally, when these beta cells were tested in a mouse model of type 1 diabetes, the animals’ blood sugar was brought under control within about two weeks.
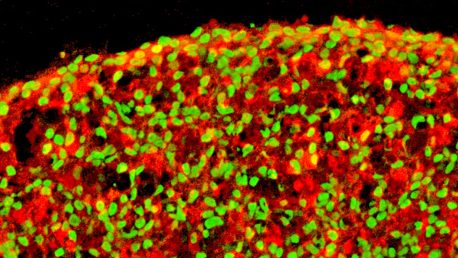
Click here for a high-resolution image.
“Stem cells are an extremely promising approach for developing many cell therapies, including better treatments for type 1 diabetes,” says Salk Professor Juan Carlos Izpisua Belmonte, the paper’s senior author. “This method for manufacturing large numbers of safe and functional beta cells is an important step forward.”
In the current work, the investigators started with human pluripotent stem cells (hPSCs). These cells, which can be derived from adult tissues (most often the skin), have the potential to become any kind of cell found in the adult body. Using various growth factors and chemicals, the investigators coaxed hPSCs into beta cells in a stepwise fashion that mimicked pancreatic development.
Producing beta cells from hPSCs in the lab is not new, but in the past the yields of these precious cells have been low. With existing methods, only about 10 to 40 percent of cells become beta cells. By comparison, techniques used to create nerve cells from hPSCs have yields of about 80 percent. Another issue is that if undifferentiated cells are left in the mix, they could eventually turn into another kind cell that would be unwanted.
“In order for beta cell-based treatments to eventually become a viable option for patients, it’s important to make these cells easier to manufacture,” says co-first author Haisong Liu, a former member of the Belmonte lab. “We need to find a way to optimize the process.”
To address the problem, the researchers took a stepwise approach to create beta cells. They identified several chemicals that are important for inducing hPSCs to become more specialized cells. They ultimately identified several cocktails of chemicals that resulted in beta cell yields of up to 80 percent.
They also looked at the ways in which these cells are grown in the lab. “Normally cells are grown on a flat plate, but we allowed them to grow in three dimensions,” says co-first author Ronghui Li, a postdoctoral fellow in the Belmonte lab. Growing the cells in this way creates more shared surface area between the cells and allows them to influence each other, just as they would during human development.
After the cells were created, they were transplanted into a mouse model of type 1 diabetes, The model mice had a modified immune system that would not reject transplanted human cells. “We found that within two weeks these mice had a reduction of their high blood sugar level into normal range,” says co-first author Hsin-Kai Liao, a staff researcher in the Belmonte lab. “The transplanted hPSC-derived beta cells were biologically functional.”
The researchers will continue to study this technique in the lab to further optimize the production of beta cells. More research is needed to assess safety issues before clinical trials can be initiated in humans. The investigators say the methods reported in this paper may also be useful for developing specialized cells to treat other diseases.
Other authors included Chao Wang, Yang Yu, Lei Shi, and Jiameng Dan of Salk; Zheying Min of Peking University; Alberto Hayek of the University of California San Diego; and Llanos Martinez Martinez and Estrella Nuñez Delicado of Universidad Católica San Antonio de Murcia, in Spain.
This work was funded by Universidad Católica San Antonio de Murcia, Primafrio, The Larry L. Hillblom Foundation, The Moxie Foundation, and Diabetes Research Connection (Project Number: 15; Institution Project Number: Liu-DRC-2019).
JOURNAL
Nature Communications
AUTHORS
Haisong Liu, Ronghui Li, Hsin-Kai Liao, Zheying Min, Chao Wang, Yang Yu, Lei Shi, Jiameng Dan, Alberto Hayek, Llanos Martinez Martinez, Estrella Nuñez Delicado, Juan Carlos Izpisua Belmonte
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.