
January 10, 2024
Salk researchers discover the molecule IMA1 links plant iron nutrition and immunity, providing a new potential target to help improve plant resilience
Salk researchers discover the molecule IMA1 links plant iron nutrition and immunity, providing a new potential target to help improve plant resilience
LA JOLLA—Plants and animals alike rely on iron for growth and regulation of microbiomes—collections of bacteria, fungi, and more that co-exist in places like the human gut or the soil around a plant’s roots. Plants face a special challenge when acquiring iron, since the strategies plants use to increase iron availability alter the root microbiome and can inadvertently benefit harmful soil-dwelling bacteria.
Now, Salk scientists have discovered how plants manage iron deficiency without helping “bad” bacteria thrive—by eliminating IMA1, the molecular signal for iron deficiency in roots at risk of bacterial attack. Additionally, they found that more IMA1 in leaves can make them more resistant to bacterial attack, suggesting the iron deficiency signaling pathway and plant immune system are deeply intertwined.

The findings were published in Nature on January 10, 2024.
“There is a long-established relationship between plant iron nutrition and bacteria,” says senior author Wolfgang Busch, professor and executive director of Salk’s Harnessing Plants Initiative. “Exploring this relationship with more nuance allowed us to find a surprising new signaling pathway that plants use to turn off iron uptake as a defense strategy against threatening bacteria that also happens to alter the plant’s immune response.”
Because bioavailable iron (iron in a state that plants and animals can use) is a relatively scarce nutrient, iron deficiency—and consequential stunted plant growth—is not uncommon. Since stopping growth is not ideal, plants have developed techniques to encourage iron absorption in low-iron environments. Unfortunately, those techniques can alter the entire microbiome around the roots and increase iron availability for not just the plant, but for the harmful bacteria living nearby, too.
To unravel the complex relationship between plant health, iron levels, and bacterial threat, the researchers turned to a small model plant called Arabidopsis thaliana. They grew the plant in low-iron and high-iron growth substrate (soil), then added fragments of flagella (little tails bacteria use to move) to mimic the presence of bacteria.
"We hypothesized there would be some sort of competition between the plant and bacteria over the iron,” says first author Min Cao, a postdoctoral researcher in Busch’s lab. “But we found that when plants feel threatened by harmful bacteria, they are willing to stop acquiring iron and stop growing—they’ll deprive themselves in order to deprive the enemy.”
When roots were exposed to flagella in low-iron environments, the plants mounted an unexpected response: Rather than the expected battle over iron between plant and bacteria, the plant immediately forfeited by eliminating the iron-deficiency signal IMA1. When roots were exposed to flagella in high-iron environments, IMA1 was not eliminated, but did not need to be expressed since iron levels were sufficient.
In plants that eliminated IMA1 in response to low iron and flagella, the researchers encountered another surprise: The more IMA1, the more resistant plant leaves were to bacterial attack. This observation led to the conclusion that iron availability and iron deficiency signaling help orchestrate the plant immune response.
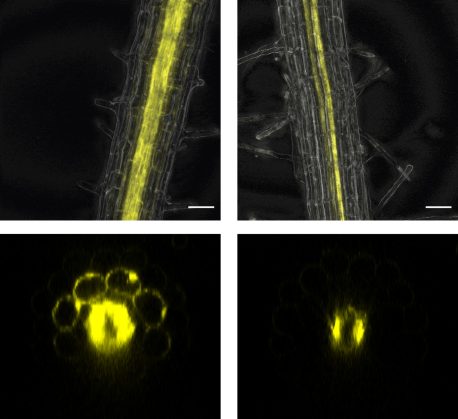
Busch believes IMA1 may be a useful target for optimizing plant immunity, which will become increasingly important as the planet’s climate continues to change and diseases begin to evolve more rapidly. Discovering that plants will halt iron uptake and arrest their growth in the face of potentially harmful bacteria is the beginning of a much longer story about plant resilience, plant and animal microbiomes, and climate change.
“Microbes determine the fate of carbon in soil, so uncovering how plants react to and impact their soil microenvironment can teach us a lot about optimizing plant carbon storage,” says Busch, who is also the Hess Chair in Plant Science at Salk. “Relatedly, understanding how plants regulate signaling and immune responses in the face of environmental scarcities, like iron deficiencies, will be crucial as scientists optimize plant health in our continually changing climate.”
In the future, the researchers will explore whether targeting IMA1 can change plant resistance to disease, and how exactly the individual cells in plant roots shut down the IMA1 signaling pathway. Learning about plant roots can teach scientists about other absorptive tissues, like the human gut, so they can better understand the intersection of mammalian microbiomes, immune systems, and iron to optimize health.
Other authors include Matthieu Pierre Platre, Ling Zhang, Tatsuya Nobori, Yingtong Chen, Wenrong He, Lukas Brent, and Joseph Ecker of Salk; Huei-Hsuan Tsai and Niko Gelder of the University of Lausanne; and Laia Armengot and Nuria Coll of the Centre for Research in Agricultural Genomics in Bellaterra, Spain.
The work was supported by the National Institutes of Health (R01GM127759, NCI CCSG: P30 014195), the Human Frontier Science Program (LT000661/2020-L), the Chapman Foundation, the Helmsley Charitable Trust, de Ministerio de Universidades, the European Union, the Ministerio de Ciencia e Innovación and Agencia Estatal de Investigació (PID2019-108595RB-I00, 10.13039/501100011033, TED2021-1311457B-I00), Taiwan’s Ministry of Science and Technology (111-2917-I-564-021), and the Centres de Recerca de Catalunya.
DOI: 10.1038/s41586-023-06891-y
JOURNAL
Nature
AUTHORS
Min Cao, Matthieu Pierre Platre, Huei-Hsuan Tsai, Ling Zhang, Tatsuya Nobori, Laia Armengot, Yintong Chen, Wenrong He, Lukas Brent, Nuria S. Coll, Joseph R. Ecker, Niko Geldner, Wolfgang Busch
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.