Professor
Molecular and Cell Biology Laboratory
J.W. Kieckhefer Foundation Chair

Our bodies are comprised of several hundred different cell types, yet each cell possesses the same genetic material. This diversity arises from selectively activating genes that are particular to each cell type, whether it is skin, liver or brain. This activation is achieved by proteins called epigenetic regulators, which work to make specific regions of our genome more or less accessible to transcription. Unlike our fixed genome, epigenetic regulation is dynamic and reversible, allowing cells to respond to developmental and environmental cues. However, mutations in epigenetic regulators are commonly found in tumors, indicating that the regulation of cell identity is an important safeguard to prevent cancer.
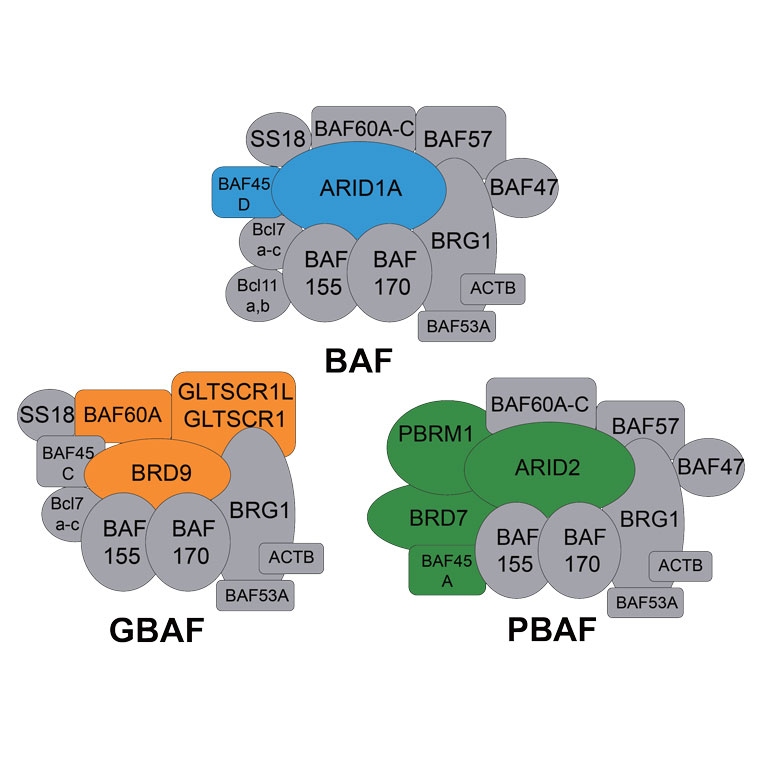
Diana Hargreaves studies a particular epigenetic regulator, the BAF complex, which uses energy to unpack and unwind DNA from structural proteins to alter DNA accessibility and, in turn, gene transcription. Her group has identified novel BAF complex variants and new roles for the BAF complex in cancer, inflammation and pluripotency (the ability of stem cells to become any cell type). She is interested in understanding how the BAF complex is targeted to specific genes, the function of the complex, and the mechanisms by which mutations in BAF subunits cause disease. Additionally, her lab explores the use of epigenetic inhibitors to disrupt BAF complex activity for therapeutic benefit. Hargreaves brings her knowledge of biochemistry and epigenetic regulation to investigate these properties in models of embryonic-stem-cell pluripotency, cellular differentiation, and macrophage activation and polarization.
Hargreaves has demonstrated that the BAF complex is an essential regulator of gene enhancers, which are important for the expression of genes involved in cell type differentiation and cancer.
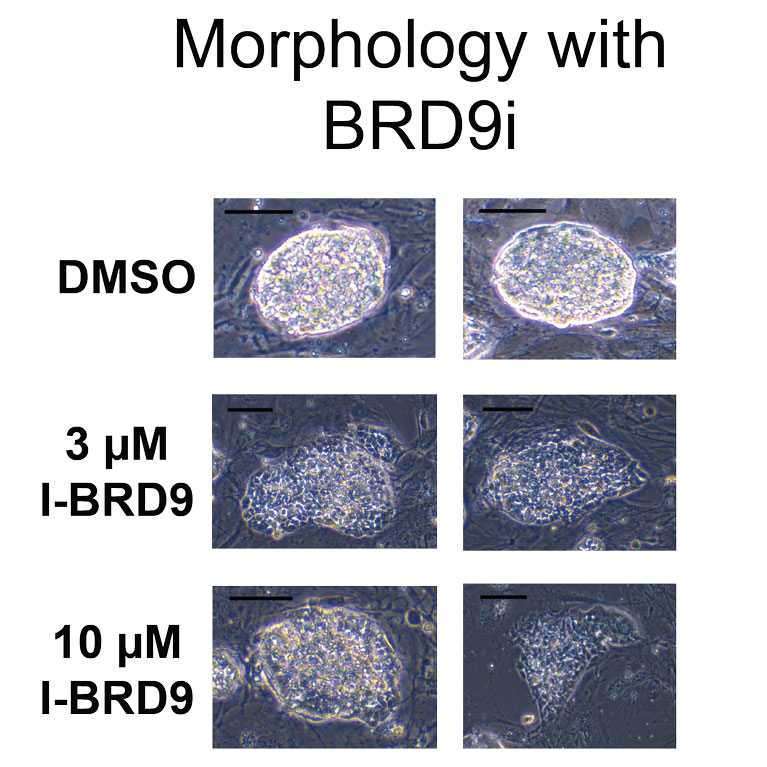
Hargreaves has discovered a non-canonical form of the BAF complex, which underlies the essential activity of the complex in stem-cell pluripotency.
By examining the role of the BAF complex in macrophages and inflammatory cells, Hargreaves is beginning to pinpoint changes in packaged DNA to better explain the role of the BAF complex and other epigenetic regulators in inflammation and tumor immunity.
BS, Chemistry and Biochemistry, Haverford College
PhD, Department of Immunobiology, Yale University
Postdoctoral Fellowship, Department of Developmental Biology, Stanford University