
December 20, 2012
Salk discovery of new role for telomeres in cellular growth may shed light on aging and age-related diseases
Salk discovery of new role for telomeres in cellular growth may shed light on aging and age-related diseases
LA JOLLA, CA—For humans to grow and to replace and heal damaged tissues, the body’s cells must continually reproduce, a process known as “cell division,” by which one cell becomes two, two become four, and so on. A key question of biomedical research is how chromosomes, which are duplicated during cell division so that each daughter cell receives an exact copy of a person’s genome, are arranged during this process.
Now, scientists at the Salk Institute have discovered a new characteristic of human cell division that may help explain how our DNA is organized in the nucleus as cells reproduce. They found that telomeres, molecular caps that protect the ends of the chromosomes, move to the outer edge of the cell’s nucleus after they have been duplicated.
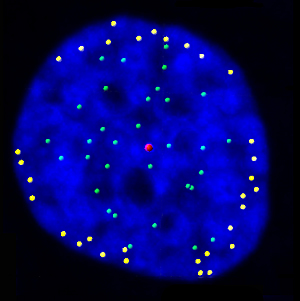
This image shows telomeres (yellow), protective caps on the ends of chromosomes, moving to the outer edge of a cell’s nucleus (blue). Salk researchers found that the telomeres anchor to the nuclear envelope after the cell duplicates its DNA during division, which may help organize the chromosomes as the cell splits into two daughter cells.
Image: Courtesy of Laure Crabbe, Ph.D. Molecular and Cell Biology Laboratory, Jamie Kasuboski, Ph.D. and James Fitzpatrick, Ph.D., Waitt Advanced Biophotonics Center.
“What we discovered is that telomeres not only protect our chromosomes, they also help organize our genetic material in the nucleus,” says Jan Karlseder, a professor in Salk’s Molecular and Cell Biology Laboratory and the Donald and Darlene Shiley Chair. “This is important, because the three-dimensional position of DNA in the nucleus influences gene expression profiles and how the genome morphs over time.”
Telomeres, a combination of proteins and DNA, are crucial in DNA replication, tumor suppression and aging. Every time a primary human cell divides, its telomeres get shorter, until critically short telomeres lead cells to self-destruct. Much of Karlseder’s research has focused on understanding telomere dynamics in order to develop ways to influence the aging process, and as a result, restrict cancer cell growth.
In addition to exploring the involvement of telomeres in premature aging diseases and interactions between the DNA damage machinery and telomeres, Karlseder studies the role of telomeres during the cell cycle. Previous studies on human cells have shown that telomeres change positions during cell division, suggesting they might also play a role in organizing DNA in the nucleus. But these studies provided only isolated snapshots of telomeres at various stages of the cell cycle.
In their new study, the Salk researchers used advanced time-lapse live-cell confocal microscopy to track telomere movement in real time throughout the cell cycle. They followed the telomeres for 20 hours in living cells by labeling them with molecules that glowed under the microscope. They also recorded the movement of chromatin, a combination of DNA and proteins that forms chromosomes.
The scientists found that the telomeres moved to the outer periphery of the nuclear envelope of each daughter cell nucleus as they assemble after mitosis, the stage of cell division during which the cell’s DNA is duplicated to provide each daughter cell with its own copy. By exploring the underlying molecular pathways, the researchers determined that interactions between two proteins, RAP1 and Sun1, seem to tether the telomeres to the nuclear envelope. Sun1 alone was also capable of attracting the telomeres to the nuclear envelope, suggesting the protein is essential for the process and that other elements might be able to replace RAP1 during tethering.
“The tethering of telomeres to the nuclear envelope may serve as an anchor point to reorganize chromatin after each cell division, so that our DNA is correctly situated for gene expression,” Karlseder says. “This tethering could also play a role in the maintenance of telomeres, thereby influencing aging, cancer development and other disorders associated with DNA damage. We plan to explore these possibilities in future experiments.”
Other Salk Institute researchers on the paper were Laure Crabbe, Anthony J. Cesare and James M. Kasuboski and James A.J. Fitzpatrick of the Waitt Advanced Biophotonics Center Core Facility.
The research was supported by the Glenn Foundation For Medical Research, the National Institutes of Health (Grant 5T32CA009370-29), the Robert John Sabo Trust and the Highland Street Foundation.
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world’s preeminent basic research institutions, where internationally renowned faculty probe fundamental life science questions in a unique, collaborative, and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer’s, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent nonprofit organization and architectural landmark.
JOURNAL
Cell Reports
AUTHORS
Laure Crabbe; Anthony J Cesare; James M Kasuboski; James A.J. Fitzpatrick; Jan Karlseder
Office of Communications
Tel: (858) 453-4100
press@salk.edu