
February 15, 2012
Scientists discover that a regulator of inflammation plays a key role in the proliferation of lung cancer cells
Scientists discover that a regulator of inflammation plays a key role in the proliferation of lung cancer cells
LA JOLLA,CA—Drugs targeting an enzyme involved in inflammation might offer a new avenue for treating certain lung cancers, according to a new study by scientists at the Salk Institute for Biological Studies.
The scientists discovered that blocking the activity of the enzyme IKK2, which helps activate the body’s inflammation response, slowed the growth of tumors in mice with lung cancer and increased their lifespan.
The findings, reported February 12 in Nature Cell Biology, suggest that drugs that hinder the ability of the enzyme to command cellular activity might prove effective as lung cancer therapies.
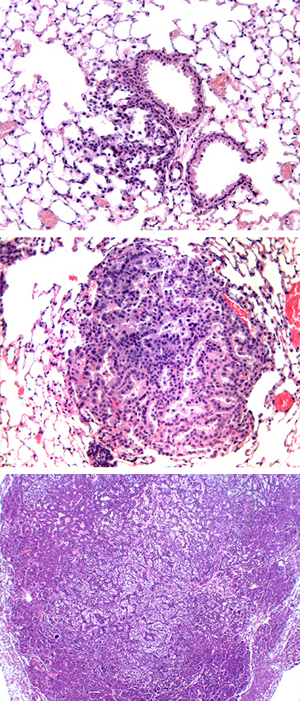
These images show the development of cancer (dark purple) in the mouse lung, initiated by lentiviral vector.
A few cancerous cells (top image) proliferate over time into a full-blown tumor (bottom image).
Image: courtesy Yifeng Xia, Salk Institute for Biological Studies
“Lung cancer is one of most lethal cancers and prognosis for patients is often poor, with only about 15 percent surviving more than 5 years,” says Inder Verma, Salk’s American Cancer Society Professor of Molecular Biology and lead author of the paper. “We developed a new method of initiating lung cancer in mice, which has properties associated with human lung cancer, and used this model to identify the role of this enzyme in cancer proliferation. We believe that this research could one day lead to therapies that improve the outlook for lung cancer patients.”
Scientists have long known that there is a link between cancer and inflammation, the body’s first line of defense against infection. Some of the same biochemical players that protect the body by controlling the inflammation response of cells can also be hijacked by genetic mutations involved in the development of cancer.
To better understand how these normally helpful components of the immune system are put to nefarious tasks in cancer cells, Verma and his colleagues developed a new method of inducing non-small-cell lung cancer in mice. This type accounts for as much as 80 percent of all lung cancer cases.
The researchers used a modified virus to insert genetic mutations into cells lining the mice’s lungs, causing the animals to develop tumors. This laid the groundwork for studies on the molecular causes of this specific cancer type that would be impossible in humans.
They then turned their attention to a protein complex, NF-KB, that initiates the inflammation response to infection by orchestrating a cell’s genetic activity. Malfunctioning regulation of NF-KB has been linked to various types of cancer, including lung cancer, but due to its many functions in the cell, drugs that directly target NF-KB would likely cause severe side effects.
To get around this limitation, the Salk researchers focused on IKK2, an enzyme that spurs NF-KB’s activity in response to stress. When they blocked IKK2 activity in the mice with lung cancer, the mice had smaller tumors and lived longer, suggesting that the enzyme is necessary for NF-KB to stimulate tumor growth.
“Now that we understand IKK2 is required for NF-KB to promote tumor growth, we hope to find ways to target its activity with drugs,” says Yifeng Xia, a postdoctoral researcher in Verma’s lab and first author on the paper. “Systemically and chronically blocking IKK2 activity is too toxic to be used in chemotherapy, but we might be able to target another molecule in the signaling pathway by which IKK2 regulates tumor growth.”
The researchers also showed that Timp-1, a gene involved in regulating cell growth, carries orders from NF-KB to tell lung cancer cells to proliferate. When they suppressed the expression of the gene, the mice with lung cancer had smaller tumors and survived longer.
“The next step is to develop antibodies or other types of drugs that can neutralize Timp-1 to abolish its pro-proliferation role in lung cancer,” says Xia.
The Salk scientists now hope to develop a mouse model of small cell lung cancer, a more aggressive form of the disease that’s been linked to smoking. They will then test whether the potential drug targets they discovered in this study would be relevant for this more deadly cancer.
The other authors on the paper were Reuben J. Shaw, a professor in Salk’s Molecular and Cell Biology Laboratory; Salk postdoctoral researcher, Narayana Yeddula, and pathologist, Mathias Leblanc; Eugene Ke, of University of California, San Diego; and Yonghui Zhang and Eric Oldfield, of the University of Illinois at Urbana-Champaign.
The study was funded by the Ellison Medical Foundation, H.N. and Frances C. Berger Foundation, Ipsen/Biomeasure, Sanofi Aventis, Leducq Foundation, National Institutes of Health, Merieux Foundation, Prostate Cancer Foundation and the U.S. Department of Defense.
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world’s preeminent basic research institutions, where internationally renowned faculty probe fundamental life science questions in a unique, collaborative, and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer’s, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent nonprofit organization and architectural landmark.
JOURNAL
Nature Cell Biology
AUTHORS
Yifeng Xia, Narayana Yeddula, Mathias Leblanc, Eugene Ke, Yonghui Zhang, Eric Oldfield, Reuben J. Shaw and Inder M. Verma
Office of Communications
Tel: (858) 453-4100
press@salk.edu