
July 8, 2010
LA JOLLA, CA-One of the most pivotal steps in evolution-the transition from unicellular to multicellular organisms-may not have required as much retooling as commonly believed, found a globe-spanning collaboration of scientists led by researchers at the Salk Institute for Biological Studies and the US Department of Energy’s Joint Genome Institute.
A comparison of the genomes of the multicellular algae Volvox carteri and its closest unicellular relative Chlamydomonas reinhardtii revealed that multicellular organisms may have been able to build their more complex molecular machinery largely from the same list of parts that was already available to their unicellular ancestors.
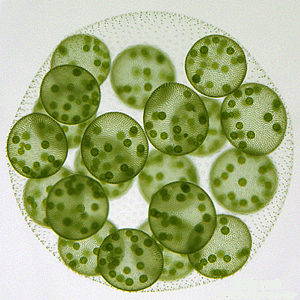
Volvox carteri
Image: Courtesy of Dr. David Kirk, Washington University, St. Louis
“If you think of proteins in terms of lego bricks Chlamydomonas already had a great lego set,” says James Umen, Ph.D., assistant professor in the Plant Molecular and Cellular Biology Laboratory at the Salk Institute. “Volvox didn’t have to buy a new one, and instead could experiment with what it had inherited from its ancestor.”
Altogether the findings, published in this week’s edition of the journal Science , suggest that very limited protein-coding innovation occurred in the Volvox lineage. “We expected that there would be some major differences in genome size, number of genes, or gene families sizes between Volvox and Chlamydomonas,” says Umen. “Mostly that turned out not to be the case.”
The evolution of multicellularity occurred repeatedly and independently in diverse lineages including animals, plants, fungi, as well as green and red algae. “This transition is one of the great evolutionary events that shaped life on earth,” says co-first author Simon E. Prochnik, Ph.D., a Computationial Scientist at the DOE Joint Genome Institute. “It has generated much thought and speculation about what makes multicellular organisms different or more complex than their unicellular ancestors.”
In most cases the switch from a solitary existence to a communal one happened so long ago-over 500 million years-that the genetic changes enabling it are very difficult to trace. An interesting exception to the rule are volvocine green algae. For them, the transition to multicellularity happened in a series of small, potentially adaptive changes, and the progressive increase in morphological and developmental complexity can still be seen in contemporary members of the group (see slide show).
Volvox, the most sophisticated member of the lineage, is believed to have evolved from a Chlamydomonas-like ancestor within the last 200 million years, making the two living organisms an appealing model to study the evolutionary changes that brought about multicellularity and cellular differentiation.
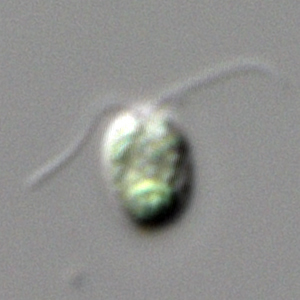
|
|
Chlamydomonas reinhardtii
The unicellular Chlamydomonas is the simplest representative of the Volvocales, a group of green algae that made the transition to multicellularity in a series of small steps. |
To gather data for the comparative genomic analysis, the researchers sequenced the 138 million base pair Volvox genome using a whole genome shotgun strategy. The genome itself is 17% larger than the previously sequenced genome of Chlamydomonas and the sequence divergence between the two is comparable to that between human and chicken.
Despite the modest increase in genome size, the number of predicted proteins turned out to be very similar for the two organisms (14,566 in Volvox vs. 14,516 in Chlamydomonas) and no significant differences could be identified in the repertoires of protein domains or domain combinations. Protein domains are parts of proteins that can evolve, function, and exist independently of the rest of the protein chain.
“This was somewhat unexpected,” explains Umen, “since innovation at the domain level was previously thought to play a role in the evolution of multicellularity in the plant and animal lineages.”
In contrast to the overall lack of innovation, protein families specific to volvocine algae, such as extracellular matrix proteins, were enriched in Volvox compared to Chlamydomonas. Each mature Volvox colony is composed of numerous flagellated cells similar to Chlamydomonas, which are embedded in the surface of a spheroid of elaborately patterned extracellular matrix (ECM) that is clearly related to the Chlamydomonas cell wall. Maybe not surprisingly, the difference in size and complexity between the Volvox extracellular matrix and Chlamydomonas cell wall is mirrored by a dramatic increase in the number and variety of Volvox genes for two major ECM protein families, pherophorins and VMPs.
Additionally, Umen and his collaborators identified an increase in the number of cyclin D proteins in Volvox, which govern cell division and may be necessary to ensure the complex regulation of cell division during Volvox development. Last but not least, Volvox adapted a few of its existing genes to acquire novel functions. Members of the pherophorin family, for one, not only help build the extracellular matrix; some subtypes evolved into a diffusible hormonal trigger for sexual differentiation.
Researchers who also contributed to this work include Alan Kuo, Uffe Hellsten, Jarrod Chapman, Astrid Terry, Jasmyn Pangilinan, Asaf Salamov, Harris Shapiro, Erika Lindquist, Susan Lucas, Igor V Grigoriev, Harris Shapiro and Daniel S. Rokhsar at U.S. Department of Energy Joint Genome Institute in Walnut Creek, Patrick Ferris at the Salk Institute for Biological Studies, Aurora Nedelcu at the University of New Brunswick in Fredericton, Canada, Arman Hallmann at the University of Bielefeld, Germany, Stephen M. Miller at the University of Maryland, Baltimore, Ichiro Nishii at the Nara Women’s University in Nara-shi, Japan, Lillian K. Fritz-Laylin at the Center for Integrative Genomics, Berkeley, Oleg Simakov at the EMBL in Heidelberg, Germany, Stefan A. Rensing at the University of Freiburg, Germany, Vladimir Kapitonov and Jerzy Jurka at the Genetic Information Research Institute in Mountain View, Jeremy Schmutz at the HudsonAlpha Institute in Huntsville, Rüdiger Schmitt at the University of Regensburg, Germany and David Kirk at Washington University in St. Louis.
-Maja Gawronska
About the Salk Institute for Biological Studies:
The Salk Institute for Biological Studies is one of the world’s preeminent basic research institutions, where internationally renowned faculty probe fundamental life science questions in a unique, collaborative, and creative environment. Focused both on discovery and on mentoring future generations of researchers, Salk scientists make groundbreaking contributions to our understanding of cancer, aging, Alzheimer’s, diabetes and infectious diseases by studying neuroscience, genetics, cell and plant biology, and related disciplines.
Faculty achievements have been recognized with numerous honors, including Nobel Prizes and memberships in the National Academy of Sciences. Founded in 1960 by polio vaccine pioneer Jonas Salk, M.D., the Institute is an independent nonprofit organization and architectural landmark.
The Salk Institute proudly celebrates five decades of scientific excellence in basic research.
Office of Communications
Tel: (858) 453-4100
press@salk.edu