
August 10, 2016
Rare genetic condition produces individuals with extremely sociable personalities but research may also shed light on biology and behavior of persons with autism and other social disorders
LA JOLLA—In a study spanning molecular genetics, stem cells and the sciences of both brain and behavior, researchers at University of California, San Diego and the Salk Institute have created a neurodevelopmental model of a rare genetic disorder that may provide new insights into the underlying neurobiology of the human social brain.
The findings are published in the August 10th online edition of Nature.
Scientists investigated Williams syndrome or WS, a rare genetic condition caused by deletion of one copy of 25 contiguous genes on chromosome 7, out of an estimated 30,000 genes in the brain. WS affects one in 10,000 people worldwide, and an estimated 20,000 Americans. The condition occurs equally in both genders and across cultures.
Click to view
Click to view addition resources on Williams syndrome»
WS results in a host of medical problems as well as a specific heart defect. Persons with the deletion typically display a distinctive face with a small, upturned nose, wide mouth, full lips and small chin and may also have dental and orthopedic problems. Neurologically, they have developmental delays, with severe spatial deficits, yet relative strengths in language use and face processing.
“An interesting aspect is the typical hyper-social predisposition,” said study co-author Ursula Bellugi, EdD, director of the cognitive neuroscience lab at Salk and an adjunct professor at UC San Diego who has studied WS for years. “Persons with the WS deletion tend to be overly friendly, overly trusting, drawn to strangers, yet anxious.”
But Bellugi said it has not been clear how genetics links to the behavioral aspects of WS. “A human model for the disease could fill in the scientific gaps and would help to understand the mechanisms behind the disorder. WS is an elegant model for being able to go across levels,” she said.
Click here for a high-resolution image.
Credit: University of California, San Diego
Co-author and Salk Professor Rusty Gage says, “By using cutting-edge stem cell techniques, we were able to, for the first time, directly observe the behavior of cells with the genetic profile of WS. This cross-disciplinary research not only suggests potential new treatments for this remarkable behavioral syndrome but could also help us to better understand the fundamental biological processes underlying social interactions.”
Senior study author Alysson Muotri, PhD, associate professor of pediatrics and cellular and molecular medicine in the UC San Diego School of Medicine, became intrigued by WS because the condition is so different from his usual research focus on autism, which is characterized by lower sociability and language skills.
“I was fascinated on how a genetic defect, a tiny deletion in one of our chromosomes, could make us friendlier, more empathetic and more able to embrace our differences,” Muotri said.
In recent years, Muotri and colleagues have created in vitro cellular models of autism using reprogrammed induced pluripotent stem cells (iPSC) derived from discarded baby teeth of children with autism, work dubbed the “tooth fairy project.” They did so again here.
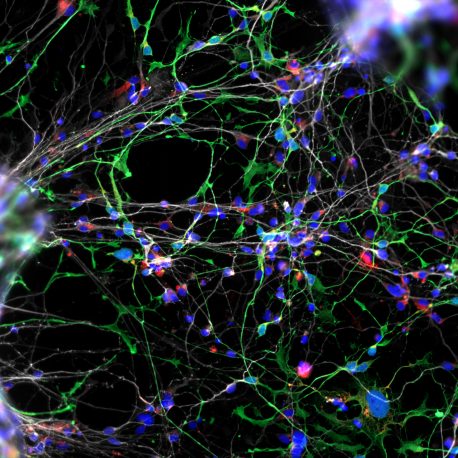
The team began with dental pulp cells extracted from teeth donated by young children with WS. The cells were reprogrammed to become neural progenitor cells able to form functional neuronal networks resembling the developing cortex of the human brain in a dish.
“We discovered that WS neural progenitor cells failed to proliferate due to high levels of cell death,” said Muotri. “And as a consequence of the lower replication of progenitor cells, WS brains have reduced cortex surface area.”
Cultured WS neurons have a distinct morphology. They are more arborized (treelike, with many dendritic branches) than neurons derived from typically developing individuals. “At the functional level, they make more synapses or connections to other neurons than what you would expect,” said Muotri. “That might underlie the WS super-social aspect and their gregarious human brain, giving insights into autism and other disorders that affect the social brain.”
The neuronal morphology was confirmed using a rare collection of WS postmortem brain tissue by Katerina Semendeferi, PhD, co-senior author and professor at UC San Diego Department of Anthropology. “One striking observation was that these cortical neurons in WS individuals are more complex than controls (typically developing children of same age). The morphological alterations that presumably appeared during WS gestation are kept postnatally.”
Muotri noted that the research represents one of the first efforts to use iPSCs and brain in-a-dish technology to generate novel insights about a disease process and not simply replicate data from other models.
But beyond that, he believes studying WS may help explain what makes humans social beings – a key development in the evolution of humanity. “It was our social power that made us a collaborative species,” said Muotri, “capable of dramatic transformation of our environment by creating poetry, music and technology.”
Co-authors include Thanathom Chailangkarn, UC San Diego and National Center for Genetic Engineering and Biotechnology, Pathum Thani, Thailand; Cleber A. Trujillo, Beatriz C. Freitas, Timothy T. Brown, Branka Hrvoj-Mihic, Lisa Stefanacci, M. Collin Ard, Kari L. Hanson, Sarah Romero and Anders M. Dale, UC San Diego; Roberto H. Herai, UC San Diego and Pontifica Universidade Catolica do Parana, Brazil; Diana X. Yu, Maria C. N. Marchetto, Cedric Bardy, Lauren McHenry, Anna Järvinen, Yvonne M. Searcy, Michelle DeWitt, Wenny Wong and Philip Lai, Fred Gage, Salk Institute for Biological Studies; Bob Jacobs, Colorado College; Li Dai and Julie R. Korenberg, University of Utah.
Funding support for this research came, in part, from the California Institute for Regenerative Medicine, the National Institutes of Health, NARSAD, Engmann Foundation, JPB Foundation, Helmsley Foundation and Royal Thai Government.
Content provided by the University of California, San Diego. Click here for UCSD press release.
JOURNAL
Nature
AUTHORS
Thanathom Chailangkarn, Cleber A. Trujillo, Beatriz C. Freitas, Branka Hrvoj-Mihic, Roberto H. Herai, Diana X. Yu, Timothy T. Brown, Maria C. Marchetto, Cedric Bardy, Lauren McHenry, Lisa Stefanacci, Anna Järvinen, Yvonne M. Searcy, Michelle DeWitt, Wenny Wong, Philip Lai, M. Colin Ard, Kari L. Hanson, Sarah Romero, Bob Jacobs, Anders M. Dale, Li Dai, Julie R. Korenberg, Fred H. Gage, Ursula Bellugi, Eric Halgren, Katerina Semendeferi & Alysson R. Muotri
Office of Communications
Tel: (858) 453-4100
press@salk.edu
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge.